Prescribing information (external link)


SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors (TKIs), and without a known T315I mutation.1
SCEMBLIX®▼ (asciminib) initiation and dosing
SCEMBLIX® (asciminib) has a once- or twice-daily oral dosing1
Recommended dosage in adult patients with Ph+ CML-CP, previously treated with ≥2 TKIs and without a known T315I mutation.1
Find out more about the efficacy of SCEMBLIX
SCEMBLIX® (asciminib) recommended dosing

SCEMBLIX should be taken at approximately the same time every day.
If a SCEMBLIX dose is missed by more than 12 hours, advise the patient to skip the dose and take the next dose as scheduled.1
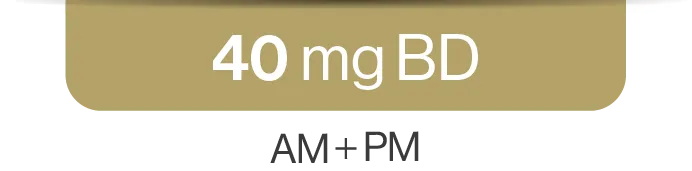
SCEMBLIX should be taken twice daily at approximately 12-hour intervals.2
If a SCEMBLIX dose is missed by more than 6 hours, advise the patient to skip the dose and take the next dose as scheduled.1

Patients should avoid food for at least 2 hours before and 1 hour after taking SCEMBLIX.1

SCEMBLIX tablets should be swallowed whole with a glass of water – patients should not break, crush or chew them.1

SCEMBLIX is available as 20 mg and 40 mg film-coated tablets.
SCEMBLIX® (asciminib) dose modification and adverse reactions
Patients changing from 40 mg twice daily to 80 mg once daily should start taking SCEMBLIX once daily approximately 12 hours after the last twice-daily dose, and then continue at 80 mg once daily.
Patients changing from 80 mg once daily to 40 mg twice daily should start taking SCEMBLIX twice daily approximately 24 hours after the last once-daily dose and then continue at 40 mg twice daily at approximately 12-hour intervals.
For the management of adverse reactions, the SCEMBLIX dose can be reduced based on individual tolerability.

Find out more about the safety profile of SCEMBLIX
SCEMBLIX should be permanently discontinued in patients unable to tolerate a total daily dose of 40 mg.1
Since there are no data available in patients with moderate or severe hepatic impairment, caution should be exercised in these patients.1
Withholding SCEMBLIX followed by potential dose reductions and/or permanent discontinuations may be required in case of thrombocytopenia and/or neutropenia, asymptomatic amylase and/or lipase elevation and non-haematological grade ≥3 adverse reactions.
SCEMBLIX dose modification schedule for the management of adverse reactions1
Adverse reaction | Dosage modification |
Thrombocytopenia and/or neutropenia | |
ANC <1.0 x 109/l and/or PLT <50 x 109/l | Withhold asciminib until resolved to ANC ≥1 x 109/l and/or PLT ≥ 50 x109/l.
For recurrent severe thrombocytopenia and/or neutropenia, withhold asciminib until resolved to ANC ≥1 x 109/l and PLT ≥50 x 109/l, then resume at reduced dose. |
Asymptomatic amylase and/or lipase elevation | |
Elevation >2.0 x ULN | Withhold asciminib until resolved to <1.5 x ULN.
|
Non-haematological adverse reactions | |
Grade 3 or higher adverse reactions* | Withhold asciminib until resolved to grade 1 or lower.
|
*Based on National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) v 4.03. | |
Please refer to the Summary of Product Characteristics (SmPC) for the detailed guidance on managing each of these adverse events and on dose modification of SCEMBLIX.1
Please refer to the SmPC for further information on changing between dosing schedules for the recommended dose of SCEMBLIX.1
SCEMBLIX, an opportunity to manage ≥3rd-line patients with a flexible dosing schedule1
Regular monitoring is important to assess treatment benefits and inform a decision to switch2,3
ANC, absolute neutrophil count; BID, twice daily; CML, chronic myeloid leukaemia; NCI CTAE, National Cancer Institute Common Terminology Criteria for Adverse Events; OD, once daily; Ph+ CML-CP, Philadelphia chromosome-positive chronic myeloid leukaemia in chronic phase; PLT, platelet; QD, once daily; SmPC, summary of product characteristics; TKI, tyrosine kinase inhibitor; ULN, upper limit of normal.
References
SCEMBLIX (asciminib) Summary of Product Characteristics.
Smith G, et al. Br J Haematol 2020;191(2):171–193.
Hochhaus A, et al. Leukemia 2020;34:966–984.
UK | January 2026 | FA-11572815
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

