SCEMBLIX®▼ (asciminib) Prescribing information (external link)
GLIVEC® (imatinib mesilate) Prescribing information (external link)


SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors (TKIs), and without a known T315I mutation.1
How can improved quality of life (QoL) support efficacy milestones in eligible patients with chronic myeloid leukaemia (CML)?
Poor medication adherence is a significant obstacle to successful CML management2
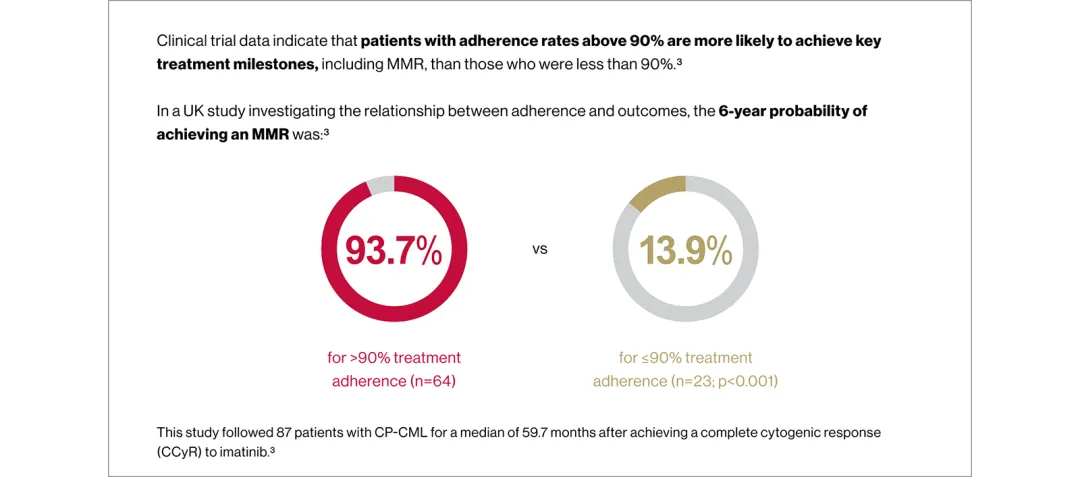
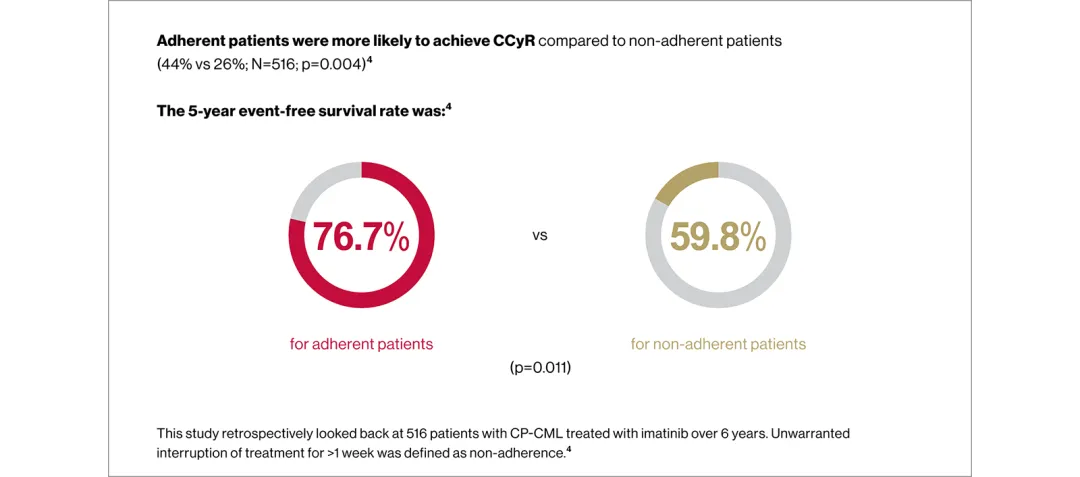
Full adherence to therapy is critical to achieving and maintaining optimal responses to CML treatment3–6
Use the arrows below to see the data from retrospective analyses of TKI adherence in patients with CML.3,4


In the ADAGIO study (n=169), on average, patients with optimal treatment responses adhered to their prescribed dose more than those with suboptimal treatment responses*2
Potential factors associated with non-compliance of treatment include:2,7–10
Belief in the treatment’s effectiveness
Burden of concomitant medication
Long-term complacency or ‘treatment fatigue’
Low health literacy
Poor communication between patients and healthcare providers
Poor treatment adherence can lead to suboptimal treatment efficacy in CML, underscoring the need for proactive management of AEs to address patient QoL3,5,11
Learn more about the unmet needs in CML
Learn more about patient monitoring and management
For further information, please refer to the Summary of Product Characteristics.
*Patients were monitored over 90 days.2
AE, adverse event; CCyR, complete cytogenic response; CML, chronic myeloid leukaemia; CP, chronic phase; MMR, major molecular response; Ph+ CML, Philadelphia chromosome-positive chronic myeloid leukaemia; QoL, quality of life; TKI, tyrosine kinase inhibitor.
References
SCEMBLIX (asciminib) Summary of Product Characteristics.
Noens L, et al. Blood 2009;113(22):5401–5411.
Marin D, et al. J Clin Oncol 2010;28:2381–2388.
Ganesan P, et al. Am J Hematol 2011:86:471–474.
Rychter A, et al. Med Oncol 2017;34:104.
Chen H, et al. Oncol Ther 2024;12:131–145.
Jabbour E, et al. Am J Hematol 2012;87:687–691.
De Almeida MH, et al. Rev Bras Hematol Hemoter 2014;36:54–59.
Abraham I and MacDonald K. Br J Cancer 2012;107:901–903.
Baryakova TH, et al. Nat Rev Drug Dis 2023;22:387–409.
DeAngelo DJ. Blood Cancer J 2012;2(10):e95.
UK | February 2026 | FA-11580465-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.