Prescribing information (external link)


SCEMBLIX®▼ (asciminib) is indicated for the treatment of adult patients with Philadelphia chromosome-positive chronic myeloid leukaemia (Ph+ CML) in chronic phase (CP), previously treated with two or more tyrosine kinase inhibitors (TKIs), and without a known T315I mutation.1
Patient monitoring and management
Are you regularly checking in on your eligible patients to understand how they are doing?
It has been suggested that both molecular response and patient-reported measures such as quality of life (QoL) are needed to determine treatment success:2
Patients with CML who do not receive frequent molecular monitoring may be at risk of disease progression3,4
Monitoring for adverse events (AEs) is essential as these can affect QoL, negatively impacting treatment adherence and leading to suboptimal outcomes3
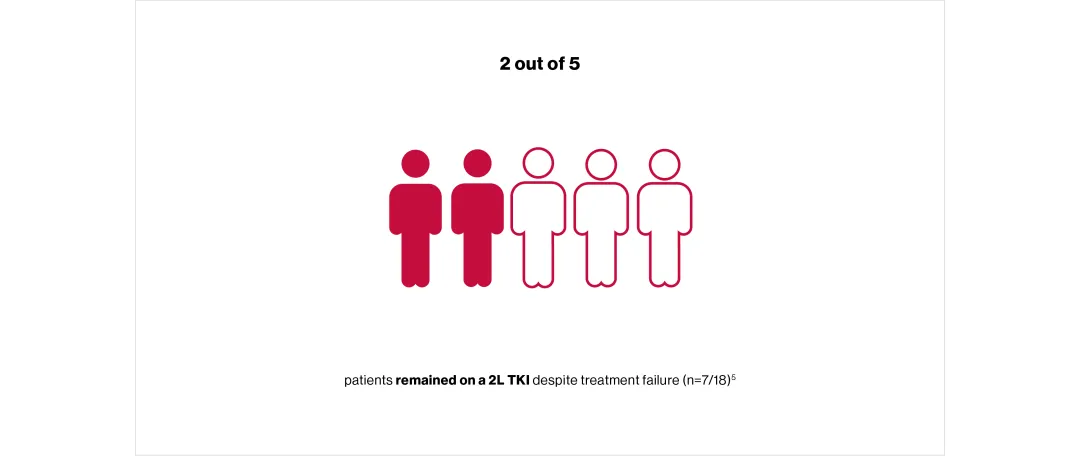
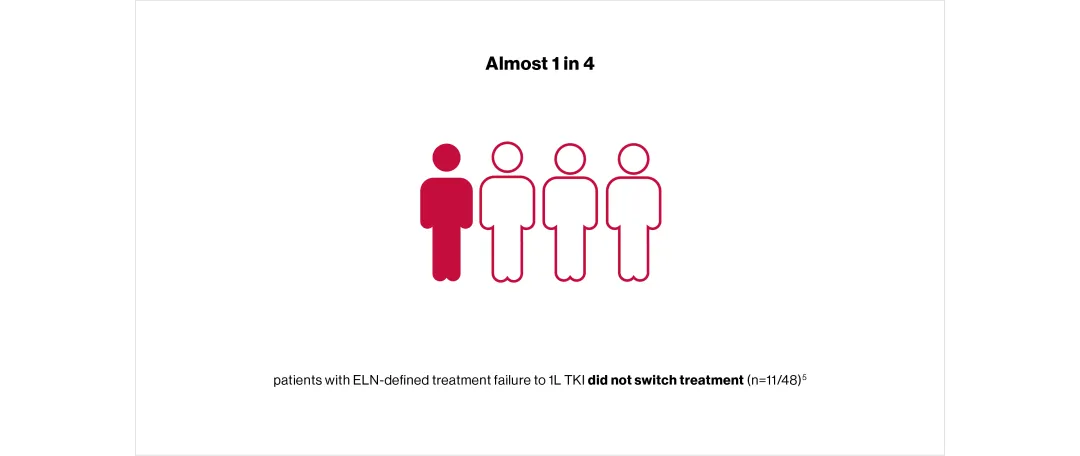
However, in the UK TARGET study, patients often did not have molecular assessments at time points recommended by the European Leukaemia Net (ELN):*5


Results from the CML survey on unmet needs (CLM SUN study) found (n=361):†2
Find out more about the CML SUN study
Don’t delay – without consistent monitoring of molecular responses and treatment-related AEs, patients requiring a treatment switch may not be identified, potentially leading to suboptimal outcomes3–6
ELN guidelines report the need for a personalised approach, acknowledging the equal importance of monitoring both toxicity and response to treatment7
Monitoring milestones of BCR-ABL1 transcript levels by the international scale at 3, 6 and 12 months is essential to determine treatment interventions, but they do not address the need to switch TKI due to AEs.7
More frequent molecular monitoring is recommended if transcript levels rise or fluctuate, or in the context of discontinuing treatment.7
Milestones for treating CML expressed as BCR-ABL1 on the international scale7
| FAVOURABLE Low risk of developing resistance: treatment switch unnecessary | WARNING Possible risk of developing resistance: treatment switch may become necessary | UNFAVOURABLE High risk of developing resistance: treatment switch preferred |
Baseline | NA | High-risk ACA, high-risk ELTS score | NA |
3 months | ≤10% | >10% | >10% if confirmed within 1–3 months |
6 months | ≤1% | >1%–10% | >10% established resistance |
12 months | ≤0.1% | >0.1%–1% | >1% |
Any time | ≤0.1% | >0.1%–1% loss of ≤0.1% MMR | Loss of a previous response, resistant BCR-ABL1 mutations, high-risk ACA |
Adapted from Apperley JF, et al. 2025.7
Even after major molecular response (MMR) is achieved, monitoring of MMR should continue every 4–6 months.7
ELN guidelines recommend SCEMBLIX as one of the first-choice options for patients resistant to their 2L treatment7
For further information, please refer to the Summary of Product Characteristics.
*TARGET was a retrospective, non-interventional study of patients with CML (N=257) in the UK who had been prescribed a 1L TKI at 21 NHS secondary and tertiary care centres. Objectives were to describe TKI treatment pathways in the UK, patient characteristics, practices for assessing and managing cardiovascular risk factors before TKI treatment, responses to 1L and 2L TKI therapy, reasons for stopping/changing TKIs, adherence to ELN 2013 recommendations and disease progression frequency and management.5
†Results from the CML SUN survey, which assessed the unmet needs of patients (n=361) with CML and their treating clinicians (n=198) in 11 countries.2 Patients ranked their top 3 most important treatment goals by line of therapy; clinicians selected any goals that they have by line of therapy.2
1L, first-line; 2L, second-line; ACA, additional chromosome abnormality; AE, adverse event; BCR-ABL1, breakpoint cluster Abelson region murine leukaemia viral oncogene homolog 1; CML, chronic myeloid leukaemia; CML SUN, CML Survey on Unmet Needs; CP, chronic phase; ELN, European Leukaemia Net; ELTS, EUTOS long-term survival; MMR, major molecular response; Ph+ CML, Philadelphia chromosome-positive chronic myeloid leukaemia; QoL, quality of life; TKI, tyrosine kinase inhibitor.
References
SCEMBLIX (asciminib) Summary of Product Characteristics.
Lang F, et al. Haematologica 2025
Chen H, et al. Oncol Ther 2024;12:131–145.
Goldberg SL, et al. Curr Med Res Opin 2013;29:1075–1082.
Milojkovic D, et al. Br J Haematol 2021;192:62–74 and supplementary data.
DeAngelo DJ. Blood Cancer J 2012;2:e95.
Apperley JF, et al. Leukemia 2025;39:1797–1813.
UK | January 2026 | FA-11555294
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.