Xolair® (omalizumab) resources
Downloadable materials to support the use of Xolair in day-to-day practice.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Indications in severe allergic asthma (SAA):1
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing immunoglobulin E (IgE) mediated asthma.
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%) as well as frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
For the full therapeutic indications of Xolair and for further safety profile information, please refer to the summary of product characteristics (SmPC), here.
To learn more about Xolair's safety profile, please click here.
In rare cases, patients on therapy with anti-asthma medicinal products, including Xolair, may present or develop systemic eosinophilia and vasculitis. These events are commonly associated with the reduction of oral corticosteroid therapy. In these patients, physicians should be alert to the development of marked eosinophilia, vasculitic rash, worsening pulmonary symptoms, paranasal sinus abnormalities, cardiac complications, and/or neuropathy. Discontinuation of omalizumab should be considered in all severe cases with the above-mentioned immune system disorders.1
In a systematic review of 42 real-world studies:2
Xolair was observed to reduce the frequency of exacerbations vs baseline up to and beyond 4 years2
Systematic review of real-word evidence on Xolair in SAA from 2008–2018 (42 studies)2
Assessed the long-term effectiveness of Xolair in terms of reducing exacerbations and symptoms2
In the INNOVATE RCT:3
83.2% of patients receiving Xolair (174/209) versus 73.8% of patients receiving placebo (155/210) did not experience a severe exacerbation over 28 weeks3
Randomised, placebo-controlled, double-blind, multicentre study (N=419)3
Definition of severe exacerbation: PEF or FEV1 <60% of personal best, requiring treatment with systemic corticosteroids3
A retrospective, real-world study (STELLAIR) demonstrated:4
Xolair effectiveness was similar in patients with ‘high’ and ‘low’ eosinophil levels4
Retrospective, real-life study (N=872)4
Low eosinophil count (<300 cells/μl): 58.1% combined responders (n=346)4
High eosinophil count (≥300 cells/μl): 58.4% combined responders (n=377)4
Definition of combined response: A combination of GETE evaluation and a ≥40% reduction in the annual exacerbation rate4
Rate of clinically significant asthma exacerbations (defined as a worsening of asthma symptoms requiring treatment with systemic corticosteroids) during the 28-week double-blind treatment phase:
Xolair was observed to reduce (with adjustment for baseline exacerbation history): 0.68 for Xolair versus 0.91 for placebo (p=0.042)
Rate ratio without adjustment for baseline exacerbation history: 0.806 (p=0.153) was not statistically significant
In the INNOVATE RCT:3
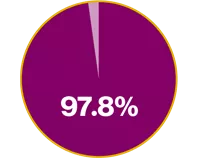
Xolair demonstrated a 97.8% improvement in QoL vs placebo3
Mean AQLQ score at baseline for Xolair vs placebo was 3.9 (1.05) and 3.9 (1.12), respectively. LSM AQLQ score at Week 28 of 0.91 vs 0.46, respectively (p<0.001) (absolute difference in overall AQLQ score: 0.45)3
Randomised, placebo-controlled, double-blind, multicentre study (N=419)3
QoL was assessed using the Juniper AQLQ at Weeks 0, 12 and 28 of the treatment phase3
In a post-hoc analysis of the INNOVATE RCT:5
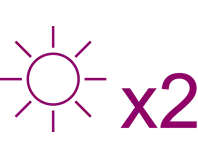
Xolair responders (n=118) experienced twice as many symptom-free days than those who received placebo (n=210): 45.8% vs 22.6% percentage symptom-free days, respectively (p<0.001)5
Post-hoc analysis of INNOVATE (N=396)5
The percentage of symptom-free days (total symptom score of 0) were compared at the last observed interval (Weeks 26–28) between treatment groups using chi-square test.5
Xolair has been on the market for over 16 years6
Over 1.3 million patient years of real-world experience in SAA and CSU6
An established safety profile including moderate data in pregnancy1
If clinically needed, the use of Xolair may be considered during pregnancy1
Store in the refrigerator between 2 and 8°C. Do not freeze. Can be left out for a total of 48 hours at 25°C.1
A randomised, multicentre, placebo-controlled, double-blind, parallel-group study in 419 patients (aged 12–75 years) with severe persistent allergic asthma who were inadequately controlled despite treatment with a high-dose ICS plus LABA, and additional controller medication if required. In the post-hoc adjustment for baseline exacerbation history, after adjustment for primary ITT population, the clinically significant asthma exacerbation rate showed a statistically significant between-group difference (p=0.042).
Primary endpoint:
Rate of clinically significant asthma exacerbations (defined as a worsening of asthma symptoms requiring treatment with systemic corticosteroids) during the 28-week double-blind treatment phase
The primary efficacy variable analysis included a post-hoc adjustment for baseline exacerbation history
Secondary endpoints included:
Severe exacerbation rate (severe exacerbation defined as PEF or FEV1 <60% of personal best, requiring treatment with systemic corticosteroids)
Rate of hospitalisation, emergency room visits and unscheduled doctor’s visits for exacerbations
Symptoms, morning PEF, rescue medication use and FEV1
QoL at Weeks 0, 12 and 28 of the treatment phase (assessed using AQLQ)
Treatment effectiveness at treatment end
A multicentre, non-interventional, retrospective, observational study using data from medicinal records of patients with SAA treated with Xolair.
Primary endpoint:
Response to Xolair at T4–6 compared with T–12 using GETE, ≥40% reduction in annual exacerbation rate and a combined response of both
Response was analysed according to blood eosinophil count (cells/µL) measured in the year prior to Xolair initiation
During allergic asthma clinical trials in adult and adolescent patients 12 years of age and older, the most commonly reported adverse reactions were headaches and injection site reactions, including injection site pain, swelling, erythema and pruritus. In clinical trials in children 6 to <12 years of age, the most commonly reported adverse reactions were headache, pyrexia and upper abdominal pain.
Infections and infestations | |
Uncommon | Pharyngitis |
Rare | Parasitic infection |
Blood and lymphatic system disorders | |
Not known | Idiopathic thrombocytopenia, including severe cases |
Immune system disorders | |
Rare | Anaphylactic reaction, other serious allergic conditions, anti-omalizumab antibody development |
Not known | Serum sickness, may include fever and lymphadenopathy |
Nervous system disorders | |
Common | Headache* |
Uncommon | Syncope, paraesthesia, somnolence, dizziness† |
Vascular disorders | |
Uncommon | Postural hypotension, flushing |
Respiratory, thoracic and mediastinal disorders | |
Uncommon | Allergic bronchospasm, coughing |
Rare | Laryngoedema |
Not known | Allergic granulomatous vasculitis (i.e., Churg-Strauss syndrome) |
Gastrointestinal disorders | |
Common | Abdominal pain upper†‡ |
Uncommon | Dyspeptic signs and symptoms, diarrhoea, nausea |
Skin and subcutaneous tissue disorders | |
Uncommon | Photosensitivity, urticaria, rash, pruritus |
Rare | Angioedema |
Not known | Alopecia |
Musculoskeletal and connective tissue disorders | |
Common | Arthralgia§ |
Rare | Systemic lupus erythematosus |
Not known | Myalgia, joint swelling |
General disorders and administration site conditions | |
Very common | Pyrexia‡ |
Common | Injection site reaction such as swelling, erythema, pain, pruritic |
Uncommon | Influenza-like illness, swelling arms, weight increase, fatigue |
*Very common in children 6 to <12 years of age.1 | |
AQLQ, asthma quality of life questionnaire; CSU, chronic spontaneous urticaria; FEV1, forced expiratory volume in 1 second; GETE, global evaluation of treatment effectiveness; ICS, inhaled corticosteroid; IgE, immunoglobulin E; ITT, intent-to-treat; LABA, long-acting beta2-agonist; LSM, least squares mean; PEF, peak expiratory flow; QoL quality of life; RCT, randomised controlled trial; SAA, severe allergic asthma; SmPC, summary of product characteristics.
References
Xolair® (omalizumab) Summary of Product Characteristics.
MacDonald KM, et al. Expert Rev Clin Immunol 2019;15(5):553–569.
Humbert M, et al. Allergy 2005;60(3):309–316.
Humbert M, et al. Eur Respir J 2018;51(5):1702523.
Humbert M, et al. Allergy 2008;63(5):592–596.
Novartis Data on File. Periodic Safety Update Report (PSUR) 2019.
UK | May 2025 | FA-11222461-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.