Contact us
Contact a Novartis representative.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Indications in severe allergic asthma (SAA):1
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing immunoglobulin E (IgE) mediated asthma.
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%) as well as frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Please refer to the Xolair Summary of Product Characteristics (SmPC) for the full therapeutic indications.1
Tools and promotional resources, created and funded by Novartis, have been designed for healthcare professional to support the use of Xolair in day‑to‑day practice.
If you have any questions please contact your local Novartis key account manager or get in touch using the ‘Contact us’ button to the left of this page.
Patients with no known history of anaphylaxis may self-inject Xolair or be injected by a caregiver from the 4th dose onwards if a physician determines that this is appropriate. The patient or the caregiver must have been trained in the correct injection technique and the recognition of the early signs and symptoms of serious allergic reactions.
Patients or caregivers should be instructed to inject the full amount of Xolair according to the instructions for use provided in the package leaflet.
Please do not share links to this website or screenshots with patients as this website is intended for healthcare professionals only.
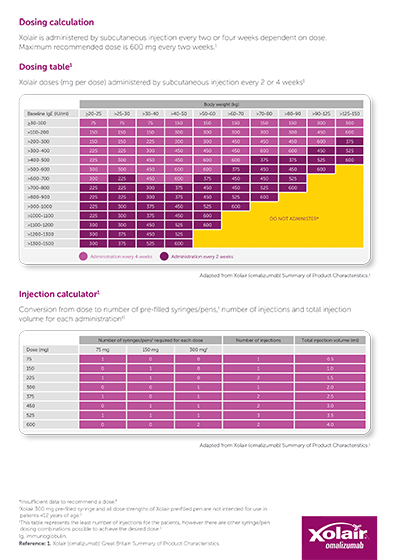
Download the dosing table and the injection guide to support you and your patients during dosing and transition to self-administration.
Please note that these resources are designed for optimal use on desktop or tablet. Functionality may be restricted on some devices, including mobile.
*To order printed materials, please contact your local Novartis representative or get in touch using the contact button to the left of this page.
FEV1, forced expiratory volume in 1 second; IgE, immunoglobulin E; SmPC, summary of product characteristics.
Reference
Xolair® (omalizumab) Summary of Product Characteristics.
UK | March 2025 | FA-11360024
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.