Xolair® (omalizumab) resources
Downloadable materials to support the use of Xolair in day-to-day practice.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Indications in severe allergic asthma (SAA):1
Xolair is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing immunoglobulin E (IgE) mediated asthma.
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%) as well as frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Please refer to the Xolair Summary of Product Characteristics (SmPC) for the full therapeutic indications.1
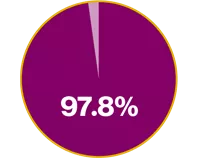
In the INNOVATE RCT, Xolair demonstrated:2
97.8% improvement in QoL vs placebo2
Mean AQLQ score at baseline for Xolair vs placebo was 3.9 (1.05) and 3.9 (1.12), respectively. LSM AQLQ overall score at Week 28 of 0.91 vs 0.46, respectively (p<0.001) (absolute difference: 0.45)
125% increase in AQLQ symptom domain score vs placebo2
LSM AQLQ symptom domain score at Week 28 of 0.90 vs 0.40, respectively (p<0.001) (absolute difference in overall AQLQ score: 0.50)
Randomised, placebo-controlled, double-blind, multicentre study (N=419)2
QoL was assessed using the Juniper AQLQ at Weeks 0, 12 and 28 of the treatment phase
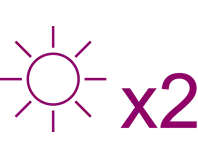
In a post-hoc analysis of the INNOVATE RCT:3
It was observed that Xolair responders experienced twice as many symptom-free days than those who received placebo (p<0.001)3
Xolair responders
(n=118)
Placebo
(n=210)
Post-hoc analysis of INNOVATE (N=396)3
The percentage of symptom-free days (total symptom score of 0) were compared at the last observed interval (Weeks 26–28) between treatment groups using chi-square test
In the PERSIST prospective, open-label, observational, multicentre study of Xolair in SAA:4
82% of ITT patients (n=163) experienced an increase in total AQLQ scores of ≥0.5 points at 16 weeks (p<0.001). AQLQ total mean score (SD) at baseline was 3.24 (1.21)4
Observational, open-label, multicentre study (N=158)4
Subjective asthma-related QoL was assessed at baseline and at 16 and 52 weeks, using the Juniper AQLQ
A randomised, multicentre, placebo-controlled, double-blind, parallel-group study in 419 patients (aged 12–75 years) with severe persistent allergic asthma who were inadequately controlled despite treatment with a high-dose ICS plus LABA, and additional controller medication if required.
The primary efficacy variable analysis included a post-hoc adjustment for baseline exacerbation history. After adjustment for the primary ITT population, the clinically significant asthma exacerbation rate showed a statistically significant between-group difference (0.68 with Xolair vs 0.91 with placebo; p=0.042).
Primary endpoint:
Rate of clinically significant asthma exacerbations (defined as a worsening of asthma symptoms requiring treatment with systemic corticosteroids) during the 28-week double-blind treatment phase
Secondary endpoints included:
Severe exacerbation rate (severe exacerbation defined as PEF or FEV1 <60% of personal best, requiring treatment with systemic corticosteroids)
Rate of hospitalisation, emergency room visits, and unscheduled doctor’s visits for exacerbations
Symptoms, morning PEF, rescue medication use and FEV1
Quality of life at Weeks 0, 12 and 28 of the treatment phase (assessed using AQLQ)
Treatment effectiveness at treatment end
Prospective, open-label, observational, multicentre 52-week study in 158 patients (aged ≥12 years) with severe persistent allergic asthma despite treatment with an ICS and a LABA.
Primary objectives:
Describe the patients who, in the treating physician’s best clinical judgement, were being treated with Xolair
Determine the 16- and 52-week effectiveness of Xolair as an add-on therapy by measuring the proportion of patients:
improving in 2005 GINA classification
with good/excellent GETE rating
achieving ≥0.5 point improvement in total AQLQ score
severe exacerbation-free (severe exacerbation defined as exacerbation during which the patient required a systemic corticosteroid, or emergency room visit or hospitalisation for the exacerbation)
improving in EQ-5D utility (52 weeks only)
Describe the treatment patterns involving add-on Xolair treatment
Describe the safety and tolerability of treatment with Xolair when used in a pragmatic trial
AQLQ, asthma quality of life questionnaire; EQ-5D, EuroQol 5-Dimensions; ICS, inhaled corticosteroid; IgE, immunoglobulin E; ITT, intent-to-treat; FEV1, forced expiratory volume in 1 second; GETE, global evaluation of treatment effectiveness; GINA, Global Initiative for Asthma; LABA, long-acting beta2-agonist; LSM, least squares mean; PEF, peak expiratory flow; QoL, quality of life; RCT, randomised controlled trial; SAA, severe allergic asthma; SmPC, summary of product characteristics.
References
Xolair® (omalizumab) Summary of Product Characteristics.
Humbert M, et al. Allergy 2005;60(3):309–316.
Humbert M, et al. Allergy 2008;63(5):592–596.
Brusselle G, et al. Respir Med 2009;103(11):1633–1642.
UK | May 2025 | FA-11359989-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.