Xolair® (omalizumab) resources
Downloadable materials to support the use of Xolair in day-to-day practice.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Indications in severe allergic asthma (SAA):1
Xolair® (omalizumab) is indicated in adults, adolescents and children (6 to <12 years of age).
Xolair treatment should only be considered for patients with convincing immunoglobulin E (IgE) mediated asthma.
Adults and adolescents (12 years of age and older)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and who have reduced lung function (FEV1 <80%) as well as frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Children (6 to <12 years of age)
Xolair is indicated as add-on therapy to improve asthma control in patients with severe persistent allergic asthma who have a positive skin test or in vitro reactivity to a perennial aeroallergen and frequent daytime symptoms or night-time awakenings and who have had multiple documented severe asthma exacerbations despite daily high-dose inhaled corticosteroids, plus a long-acting inhaled beta2-agonist.
Please refer to the Xolair Summary of Product Characteristics (SmPC) for the full therapeutic indications.1
Xolair is recommended as an option for treating severe persistent confirmed allergic IgE‑mediated asthma as an add‑on to optimised standard therapy in people aged ≥6 years who need continuous or frequent treatment with oral corticosteroids (defined as ≥4 courses in the previous year).2
Xolair must be made available with the discount agreed in the patient access scheme. Optimised standard therapy is defined as a full trial of and, if tolerated, documented compliance with inhaled high‑dose corticosteroids, long‑acting beta2 agonists, leukotriene receptor antagonists, theophyllines, oral corticosteroids, and smoking cessation if clinically appropriate. People currently receiving Xolair whose disease does not meet the criteria above should be able to continue treatment until they and their clinician consider it appropriate to stop.2
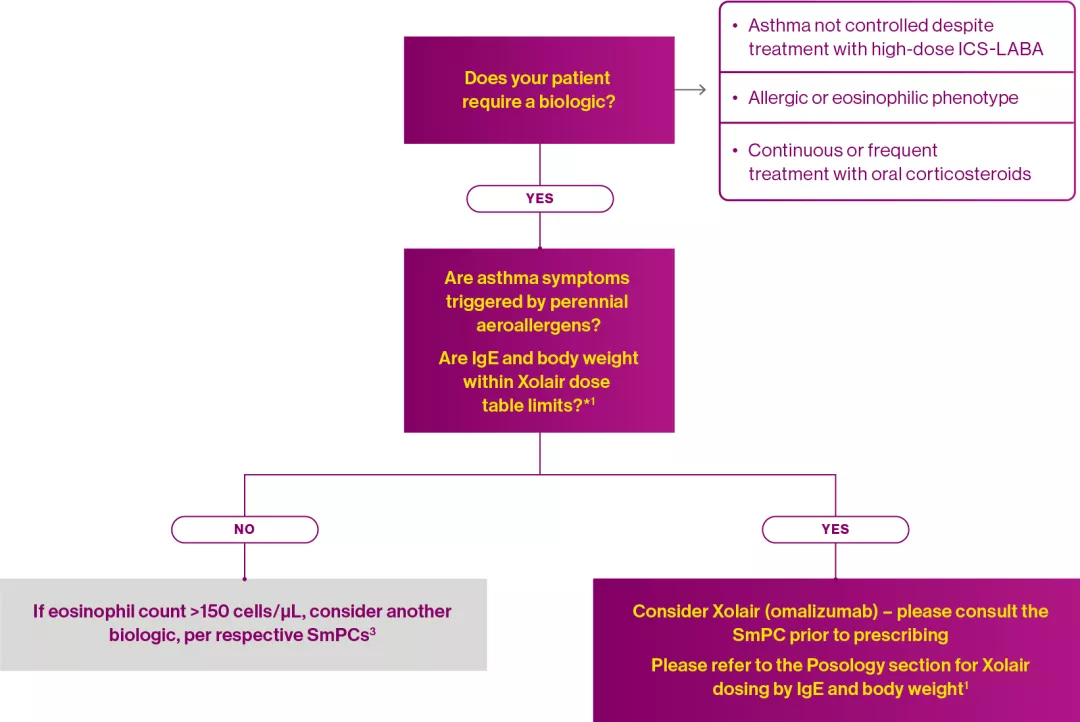
This is supported by the review by Viswanathan & Busse (2020), who proposed a decision-making algorithm for selecting a biologic for the treatment of severe asthma:3
Adapted from Xolair® Summary of Product Characteristics1 and Viswanathan RK & Busse WW 2020.3
*Patients whose baseline IgE levels or body weight in kg are outside the limits of the dose table in the Xolair SmPC should not be given Xolair.1
FEV1, forced expiratory volume in 1 second; ICS, inhaled corticosteroid; IgE, immunoglobulin E; LABA, long-acting beta2-agonist; NICE, National Institute for Health and Care Excellence; SAA, severe allergic asthma; SmPC, summary of product characteristics.
References
Xolair® (omalizumab) Summary of Product Characteristics.
National Institute for Health and Care Excellence. Omalizumab for treating severe persistent allergic asthma (TA278). Available at: https://www.nice.org.uk/guidance/ta278 [Accessed May 2025].
Viswanathan RK & Busse WW. Ann Allergy Asthma Immunology 2020;125(2);137–149.
UK | May 2025 | FA-11360014-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.