Prescribing information (external link)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-10/lutathera-home-banner-hcp-2700_v2.jpg.webp?itok=tWBpmfKb)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-10/lutathera-home-banner-hcp-1000_v2.jpg.webp?itok=KHJWRczF)
LUTATHERA® (lutetium [177Lu] oxodotreotide)
LUTATHERA® is indicated for the treatment of unresectable or metastatic, progressive, well-differentiated (G1 and G2), somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.1
LUTATHERA® Summary of Product Characteristics (SmPC) can be found here
Patients with GEP-NETs are often diagnosed too late2–5
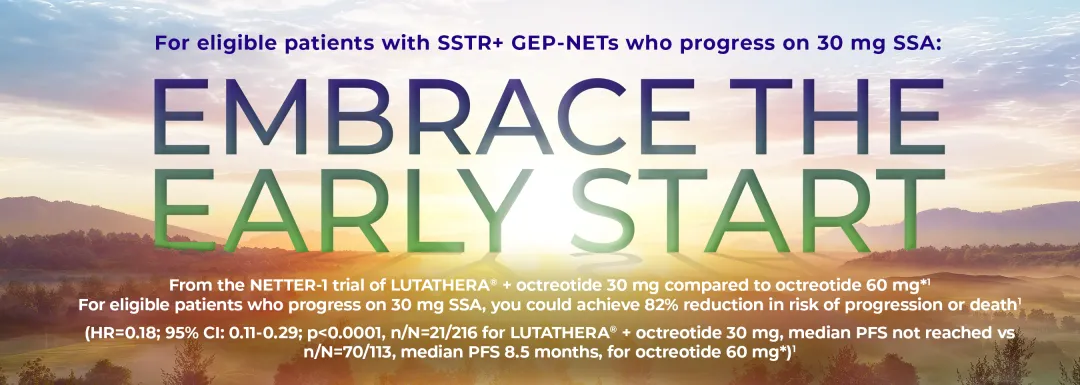
The NETTER-1 trial was a Phase III multicentre, stratified, open-label, randomised, comparator-controlled, parallel-group study comparing the effects of LUTATHERA® + octreotide 30 mg† vs octreotide 60 mg* in patients with inoperable, progressive, SSTR-positive, midgut carcinoid tumours. The primary endpoint of the trial was PFS. Secondary endpoints included overall response rate, overall survival, time to progression, safety and tolerability and health-related quality of life.1
*Please note that octreotide 60 mg is NOT the licensed dose. Scientific opinion from published literature and clinical opinion supported the use of up to 60 mg octreotide as standard of care when lower doses are inadequate.6
†Long-acting octreotide 30 mg was replaced by short-acting octreotide in the 4-week interval before LUTATHERA® administration, as long-acting SSAs should be avoided within 30 days prior to receiving LUTATHERA®. Please see the SmPC for further information on SSAs.1
Most GEP-NET patients are diagnosed late, resulting in increased burden, reduced therapeutic options and worse survival outcomes3–5
Is it time to initiate LUTATHERA®?
Here you can find a range of information about LUTATHERA®, including:
CI, confidence interval; GEP-NET, gastroenteropancreatic neuroendocrine tumour; HR, hazard ratio; PFS, progression-free survival; RLT, radioligand therapy; SmPC, summary of product characteristics; SSA, somatostatin analogue; SSTR, somatostatin receptor.
References
LUTATHERA® Summary of Product Characteristics.
Uri I & Grozinsky-Glasberg S. Clin Diabetes Endocrinol 2018;4:16.
Kos-Kudła B, et al. Contempt Oneal (Pozn) 2017; 21(2):115–122.
Keizer AC, et al. Int J Endocrin Oncol 2016;3:33–39.
Diez M, et al. Ann Gastroenterol 2013;26(1):29–36.
Broder MS, et al. World J Gastroenterol 2015;21(6):1945–1955.
Frilling A, et al. Endocr Re lat Cancer 2012;19(5):R 163–185.
Wolin E, et al. Pancreas 2017;46(5):639–647.
Alexandraki Kl & Kaltsas G. Endocrine 2012;41(1):40–52.
Shah MH, et al. J Natl Compr Cane Netw 2018;16(6):693–702.
UK | January 2026 | FA-11462529-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.