Prescribing information (external link)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-2700_0.png.webp?itok=KiWg7nHk)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-1000_0.png.webp?itok=cL3k8yXL)
Diagnosis and treatment of neuroendocrine tumours (NETs)
LUTATHERA® (lutetium [177Lu] oxodotreotide) is indicated for the treatment of unresectable or metastatic, progressive, well-differentiated (G1 and G2), somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.1
LUTATHERA® Summary of Product Characteristics (SmPC) can be found here
Diagnosis of NETs
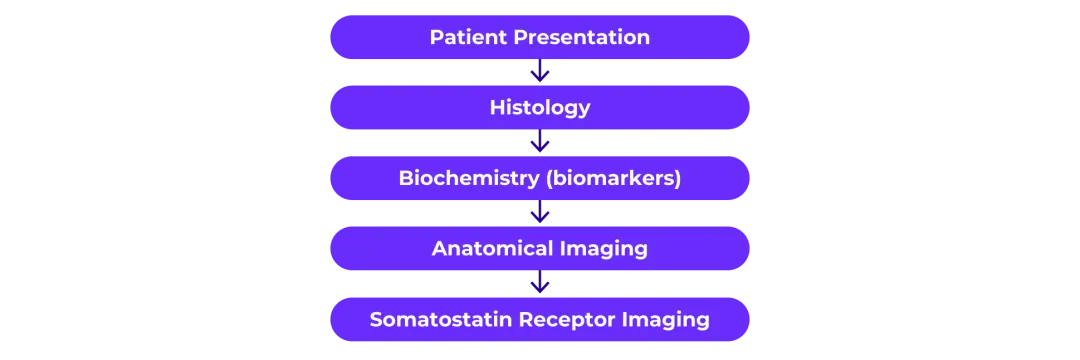
Several tools, measures and imaging techniques are used to diagnose NETs; they often follow a logical order starting with patient presentation (although not all of the following diagnostic techniques are used in all patients).2,3
Diagnosis can be confirmed by assessing biomarkers that are characteristic of NETs:4
Chromogranin A (CgA) – Produced by a wide range of GEP-NETs, bronchopulmonary-NETs and other NETs5–7
Pancreatic polypeptide (PP) – Plasma PP is elevated in patients with pancreatic and colonic NETs8–10
5-hydroxyindoleacetic acid (5-HIAA) – Midgut NETs often secrete serotonin, leading to elevation of the metabolite 5-HIAA in blood and urine9,11
Insulin and gastrin are two other biomarkers that can be used to aid diagnosis of NETs11
Treatment of NETs
Curative surgery is the primary aim of treatments, but this is only achievable in a minority of patients. For the remainder, goals of therapy are to control symptoms, prevent tumour growth and extend survival. Several classes of treatment can be used to achieve these goals, a summary of the therapies is as follows:12,13
*Everolimus is indicated for the treatment of unresectable or metastatic, well- or moderately-differentiated NETs of pancreatic origin in adults with progressive disease and (grade 1 or grade 2) non-functional NETs of gastrointestinal or lung origin in adults with progressive disease. Sunitinib is indicated for the treatment of unresectable or metastatic, well-differentiated pancreatic NETs with disease progression in adults.18,19
Afinitor (everolimus) Prescribing information (external link)
Treatment of NETs – RLT
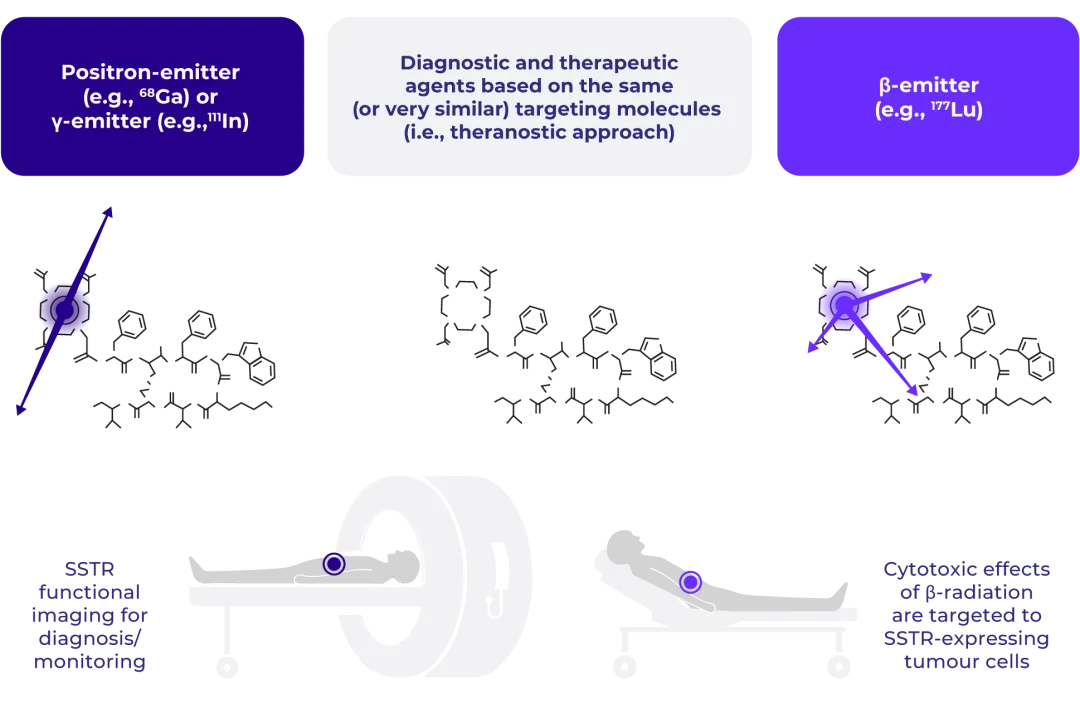
Patient eligibility for LUTATHERA® is dependent on demonstration of adequate tumoural uptake of an imaging radiotracer that also binds SSTRs.
Before starting treatment with LUTATHERA®, SSTR imaging (scintigraphy or positron emission tomography [PET]) must confirm the overexpression of these receptors in the tumour tissue with the tumour uptake at least as high as normal liver uptake.
The coupling of SSTR imaging with delivery of RLT to patients with SSTR-positive tumours is an example of what is known as a theranostic approach.24
SomaKit TOC® (edotreotide) (40 micrograms kit for radiopharmaceutical preparation) indication: This medicinal product is for diagnostic use only. After radiolabelling with gallium (68Ga) chloride solution, the solution of gallium (68Ga) edotreotide obtained is indicated for PET imaging of SSTR overexpression in adult patients with confirmed or suspected well-differentiated GEP-NET for localizing primary tumours and their metastases.25
SomaKit TOC Prescribing information (external link)
Please refer to the SmPCs for more information about LUTATHERA®, SomaKit TOC and Afinitor®.
†Streptozocin is indicated for the systemic treatment of adult patients with inoperable, advanced or metastatic, progressive and/or symptomatic, well-differentiated, G1 or G2 NETs of pancreatic origin, in combination with 5-fluorouracil.20
‡Telotristat is indicated for the treatment of carcinoid syndrome diarrhoea in combination with SSA therapy in adults inadequately controlled by SSA therapy.23
5-HIAA, 5-hydroxyindoleacetic acid; CgA, chromogranin A; GEP-NET, gastroenteropancreatic neuroendocrine tumour; PP, pancreatic polypeptide; RLT, radioligand therapy; SmPC, summary of product characteristics; SSA, somatostatin analogue; SSTR, somatostatin receptor.
References
LUTATHERA® Summary of Product Characteristics.
Raphael M, et al. CMAJ 2017;189:E398–E404.
Oronsky B, et al. Neoplasia 2017;19:991–1002.
Perren A, et al. Neuroendocrinology 2017;105:196–200.
Öberg K, et al. Lancet Oncol 2015;16:e435–e446.
Gut P, et al. Arch Med Sci 2016;12:1–9; 4.
Nobels FR, et al. J Clin Endocrinol Metab 1997;82:2622–2628.
Modlin IM, et al. Lancet Oncol 2008;9:61–72.
Modlin IM, et al. Neuroendocrinology 2014;100:265–277.
Aluri V & Dillon JS. Endocrinol Metab Clin North Am 2017;46:669–677.
Öberg K, et al. Neuroendocrinology 2017;105:201–211.
Ramage JK, et al. Gut 2012;61:6–32.
Nigri G, et al. World J Surg Oncol 2018;16:142.
Öberg KE. Ann Oncol 2010;21(Suppl 7):vii72–80.
Narayanan S, et al. Compr Canc Netw 2015;13:109–117.
Chung C. Am J Health Syst Pharm 2016;73:1729–1744.
Harring TR, et al. Int J Hepatol 2011;154541.
Afinitor® Summary of Product Characteristics.
Sunitinib® Summary of Product Characteristics.
Streptozocin® Summary of Product Characteristics.
Strosberg JR, et al. Cancer 2011;117:268–275.
Kulke MH, et al. J Clin Oncol 2006;24:401–406.
Telotristat Summary of Product Characteristics.
Navalkissoor S, et al. J Clin Med (Lond) 2017;17:462–468.
SomaKit® Summary of Product Characteristics.
UK | January 2026 | FA-11462548-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.