Prescribing information (external link)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-2700_0.png.webp?itok=KiWg7nHk)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-1000_0.png.webp?itok=cL3k8yXL)
Dosing and administration
LUTATHERA® (lutetium [177Lu] oxodotreotide) is indicated for the treatment of unresectable or metastatic, progressive, well-differentiated (G1 and G2), somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.1
LUTATHERA® Summary of Product Characteristics (SmPC) can be found here
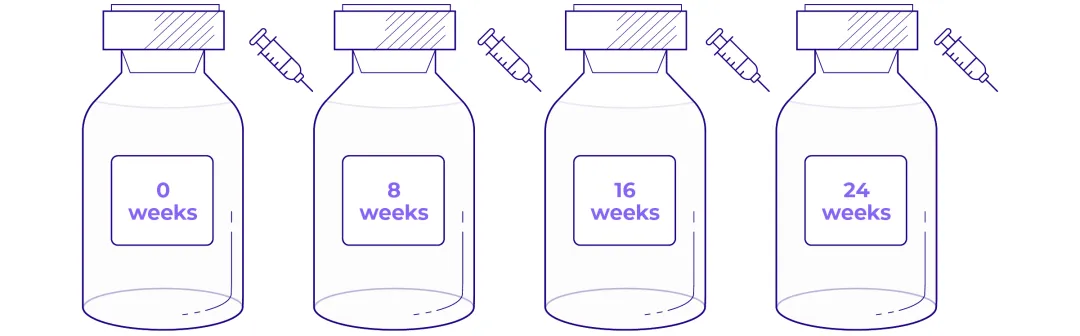
Defined, 4-dose treatment regimen1
Four doses, with a recommended treatment interval of 8 weeks (±1 week) between each administration.1
Intervals between infusions are extendable to 16 weeks in case of dose-modifying toxicity, allowing for flexibility in the regimen if needed1
Administration of LUTATHERA®*1

Adapted from LUTATHERA® SmPC.1
Do not administer long-acting somatostatin analogues (SSAs) within 30 days prior to LUTATHERA® administration. Administer short-acting SSAs as needed, but discontinue at least 24 hours prior to initiating LUTATHERA®
The gravity method, the peristaltic pump method or the syringe pump method may be used for administration of the recommended dose. Treating healthcare professionals may use other methods deemed appropriate, particularly when dose reduction is required. Radiation safety precautions must be considered regardless of the administration method used.1
Treatment monitoring¹
Before each administration and during the treatment with LUTATHERA®, laboratory tests are required to assess the patient’s condition and adapt the therapeutic protocol as necessary (dose, infusion interval, number of infusions) (see dose modifications section).
The minimum laboratory tests needed before each infusion are:
Haematology (Hb, white blood cell count with differential counts, platelet count)
Kidney function (serum creatinine and creatinine clearance by Cockcroft-Gault formula)
Liver function (ALT, AST, serum albumin, INR and bilirubin)
These laboratory tests should be performed at least once in the 2 to 4 weeks prior to administration, and shortly before administration. It is also recommended to perform these tests every 4 weeks for at least 3 months after the last infusion of LUTATHERA® and every 6 months thereafter, in order to be able to detect possible delayed adverse reactions. Dosing may need to be modified based on the test results (see dose modifications section).
Dose modification¹
Management of severe or intolerable adverse drug reactions may require temporary dose interruption (extension of the dosing interval from 8 weeks up to 16 weeks), dose reduction or permanent discontinuation of treatment with LUTATHERA® (see table and figure below).
Recommended dose modifications of LUTATHERA® for adverse drug reactions
Adverse drug reaction | Severity of adverse drug reaction | Dose modification |
Thrombocytopenia | First occurrence of:
| Withhold dose until complete or partial resolution (grade 0 to 1). Resume LUTATHERA® at 3700 MBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in grade 2, 3 or 4 thrombocytopenia, administer LUTATHERA® at 7400 MBq (200 mCi) as next dose. Permanently discontinue LUTATHERA® for grade 2 or higher thrombocytopenia requiring a dosing interval beyond 16 weeks. |
Recurrent grade 2, 3 or 4 | Permanently discontinue LUTATHERA®. | |
Anaemia and neutropenia | First occurrence of anaemia:
First occurrence of neutropenia:
| Withhold dose until complete or partial resolution (grade 0, 1 or 2). Resume LUTATHERA® at 3700 MBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in grade 3 or 4 anaemia or neutropenia, administer LUTATHERA® at 7400 MBq (200 mCi) as next dose. Permanently discontinue LUTATHERA® for grade 3 or higher anaemia or neutropenia requiring a dosing interval beyond 16 weeks. |
Recurrent grade 3 or 4 | Permanently discontinue LUTATHERA®. | |
Renal toxicity | First occurrence of:
| Withhold dose until resolution or return to baseline. Resume LUTATHERA® at 3700 MBq (100 mCi) in patients with resolution or return to baseline. If reduced dose does not result in renal toxicity, administer LUTATHERA® at 7400 MBq (200 mCi) as next dose. Permanently discontinue LUTATHERA® for renal toxicity requiring a dosing interval beyond 16 weeks. |
Recurrent renal toxicity | Permanently discontinue LUTATHERA®. | |
Hepatotoxicity | First occurrence of:
| Withhold dose until resolution or return to baseline. Resume LUTATHERA® at 3700 MBq (100 mCi) inpatients with resolution or return to baseline. If reduced LUTATHERA® dose does not result in hepatotoxicity, administer LUTATHERA® at 7400 MBq (200 mCi) as next dose. Permanently discontinue LUTATHERA® for hepatotoxicity requiring a dosing interval beyond 16 weeks. |
Recurrent hepatotoxicity | Permanently discontinue LUTATHERA®. | |
Any other CTCAE† grade 3 or grade 4 adverse drug reaction‡ | First occurrence of grade 3 or 4 | Withhold dose until complete or partial resolution (grade 0 to 2). Resume LUTATHERA® at 3700 MBq (100 mCi) in patients with complete or partial resolution. If reduced dose does not result in grade 3 or 4 toxicity, administer LUTATHERA® at 7400 MBq (200mCi) as next dose. Permanently discontinue LUTATHERA® for grade 3 or higher adverse drug reaction requiring a dosing interval beyond 16 weeks. |
Recurrent grade 3 or 4 | Permanently discontinue LUTATHERA®. |
Adapted from LUTATHERA® Summary of Product Characteristics.1
Overview of instructions for dose modifications
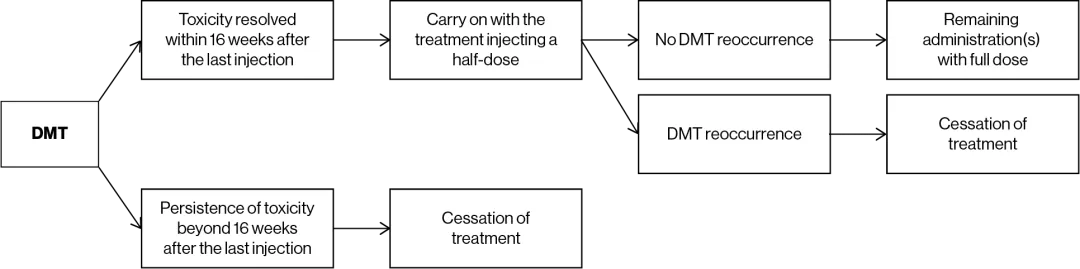
Adapted from LUTATHERA® Summary of Product Characteristics.1
Other reasons to consider temporary dose interruption of LUTATHERA® include occurrence of an intercurrent disease (e.g., urinary tract infection), which the physician considers could increase the risks associated with LUTATHERA® administration and which should be resolved or stabilised for treatment to resume, or major surgery, in the event of which treatment should be withheld for 12 weeks after the date of surgery.
Premedications and concomitant medications1
SSAs1
Amino acid solution1
For renal protection, an amino acid solution must be administered intravenously over 4 hours. The infusion of the amino acid solution should start 30 minutes prior to the start of LUTATHERA® infusion.
The amino acid solution can be prepared as a compounded product, in compliance with the hospital’s sterile medicinal product preparation good practices and according to the composition specified below:
Compound | Amount |
L-lysine HCl | 25 g§ |
L-arginine HCl | 25 g¶ |
Sodium chloride 9 mg/mL (0.9%) solution for injection, or water for injections | 1 L |
An amino acid solution containing just L-lysine and L-arginine in the amounts specified here is considered the medicinal product of choice, due to its lower total volume to be infused and lower osmolality.
Alternatively, some commercially available amino acid solutions can be used if compliant with the specification described below:
Characteristic | Specification |
L-lysine HCl | Between 18 and 25 g‖ |
L-arginine HCl | Between 18 and 25 g** |
Volume | 1 L to 2 L |
Osmolality | <1,200 mOsmol/kg |
Antiemetics1
*Consult the SmPC before initiating treatment with LUTATHERA®.1
†CTCAE: Common Terminology Criteria for Adverse Events, National Cancer Institute.1
‡No dose modification required for haematological toxicities grade 3 or grade 4 solely due to lymphopenia.1
§Equivalent to 20.0 g L-lysine.1
¶Equivalent to 20.7 g L-arginine.1
‖Equivalent to 14.4–20.0 g L-lysine.1
**Equivalent to 14.9–20.7 g L-arginine.1
ALT, alanine aminotransferase; ANC, absolute neutrophil count; AST, aspartate aminotransferase; DMT, dose-modifying toxicity; GEP-NET, gastroenteropancreatic neuroendocrine tumour; Hb, haemoglobin; HCl, hydrochloric acid; INR, international normalised ratio; RLT, radioligand therapy; SmPC, summary of product characteristics; SSA, somatostatin analogue; SSTR, somatostatin receptor.
Reference
LUTATHERA® Summary of Product Characteristics.
UK | January 2026 | FA-11462542-2
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.