Prescribing information (external link)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-2700_0.png.webp?itok=KiWg7nHk)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-1000_0.png.webp?itok=cL3k8yXL)
Safety profile
LUTATHERA® (lutetium [177Lu] oxodotreotide) is indicated for the treatment of unresectable or metastatic, progressive, well-differentiated (G1 and G2), somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.1
LUTATHERA® Summary of Product Characteristics (SmPC) can be found here
LUTATHERA® has a generally well understood safety profile1
The overall safety profile is based on pooled data from clinical trials (NETTER-1 pivotal Phase III and ERASMUS long-term Phase I/II) and compassionate use programs.1
Please refer to the SmPC in the link above for the full safety profile and for additional information on management of vomiting and nausea prior to prescribing.
Adverse events (AEs) in NETTER-1
As of the final analysis, 76% of patients in the LUTATHERA® + octreotide 30 mg arm received all four LUTATHERA® infusions, with only 11% discontinuing treatment due to AEs.*3
The majority of grade 3/4 AEs were comparable between the LUTATHERA® + octreotide 30 mg group and octreotide 60 mg† group:2,4
The most common grade 3/4 AEs were vomiting, nausea, diarrhoea, abdominal pain, and lymphopenia, which occurred in 9% or less of patients taking LUTATHERA® + octreotide 30 mg
More patients experienced grade 3 or 4 haematologic events in the LUTATHERA® + octreotide 30 mg group vs the octreotide 60 mg† group; however, these events were transient
No new safety signals were identified after a median follow-up of >6 years, reaffirming the previously reported safety profile.2,3
†Please note that octreotide 60 mg is NOT the licensed dose. Scientific opinion from published literature and clinical opinion supported the use of up to 60 mg octreotide as SOC when lower doses are inadequate.4
Sandostatin® LAR® (octreotide acetate) is indicated for the treatment of patients with symptoms associated with functional GEP endocrine tumours e.g., carcinoid tumours with features of the carcinoid syndrome, and also for the treatment of patients with advanced NET of the midgut or of unknown primary origin where non-midgut sites of origin have been excluded.
Sandostatin Prescribing information (external link)
Haematologic events in NETTER-1
Change in haematological parameters over time2
Leukocytes mean relative change from baseline over time‡2
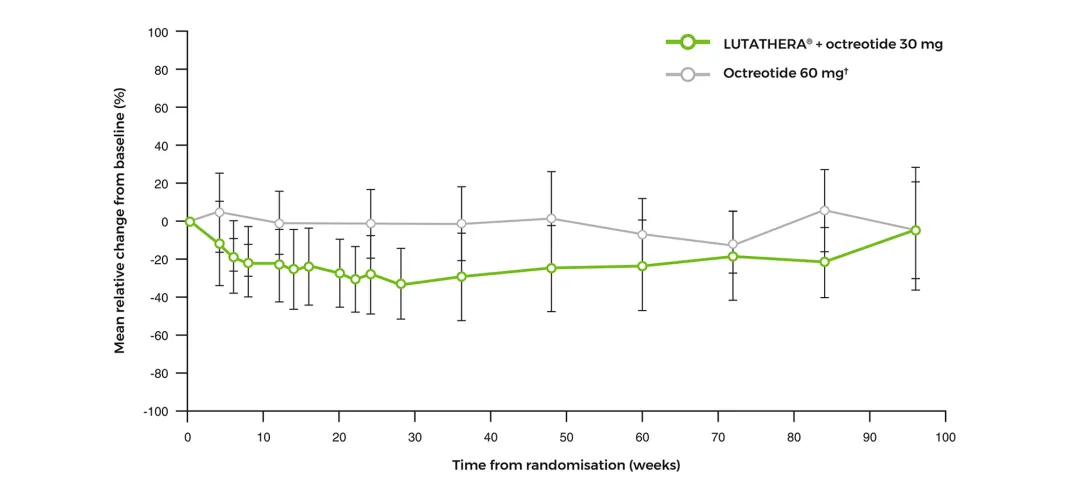
Adapted from Strosberg J, et al. 2017 (supplementary material).2
Lymphocytes mean relative change from baseline over time‡2
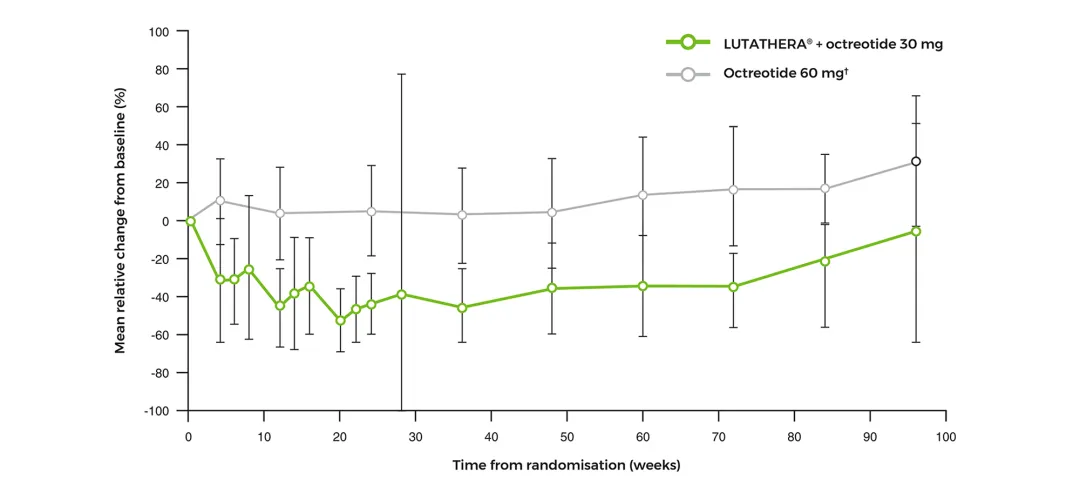
Adapted from Strosberg J, et al. 2017 (supplementary material).2
Neutrophils mean relative change from baseline over time‡2
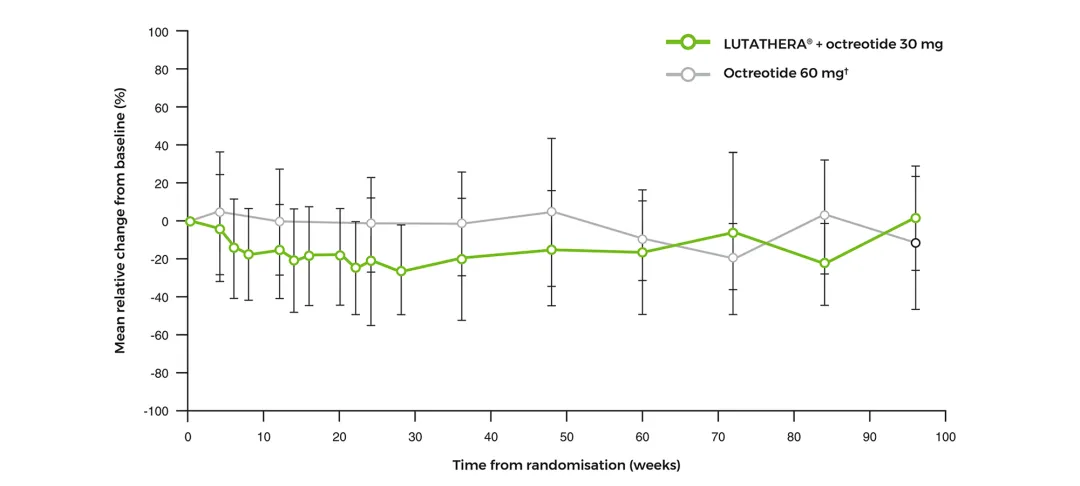
Adapted from Strosberg J, et al. 2017 (supplementary material).2
Platelets mean relative change from baseline over time‡2
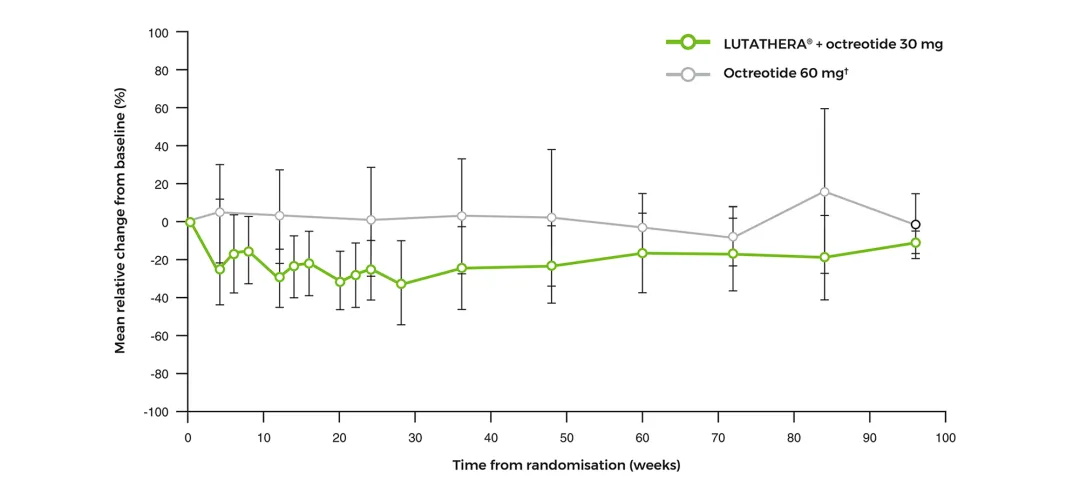
Adapted from Strosberg J, et al. 2017 (supplementary material).2
LUTATHERA® long-term safety profile§
At the time of the NETTER-1 final analysis, after a median follow-up time of over 6 years for each study arm, no new safety signals were identified.3
§Please note that late-onset MDS and acute leukaemia have been observed after treatment with LUTATHERA®. The aetiology of these therapy-related secondary myeloid neoplasms is unclear. Please refer to the SmPC for more information.1
¶Renal function as determined by serum creatinine and calculated creatinine clearance using Cockcroft-Gault formula must be assessed at baseline, during and for at least the first year after treatment. Please refer to the SmPC for more information.1
Please note, these are the focus of special warnings in the LUTATHERA® SmPC. Please refer to the SmPC for full safety guidance.1
Special populations¹
Elderly
No dose adjustment is required in patients aged 65 years or above as clinical experience has not identified differences in responses between the elderly and younger patients. However, since increased risk of presenting with haematotoxicity has been described in elderly patients (≥70 years old), close follow-up allowing for prompt dose adaptation (DMT) in this population is advisable.
Renal impairment
Careful consideration of the activity to be administered to patients with renal impairment is required since an increased radiation exposure is possible in these patients. The pharmacokinetic profile and safety of LUTATHERA® in patients with baseline severe renal impairment (creatinine clearance <30 mL/min by Cockcroft-Gault formula) or end-stage renal disease have not been studied. Treatment with LUTATHERA® in patients with kidney failure with creatinine clearance <30 mL/min is contraindicated. Treatment with LUTATHERA® in patients with baseline creatinine clearance <40 mL/min (using Cockcroft-Gault formula) is not recommended. No dose adjustment is recommended for renally impaired patients with baseline creatinine clearance ≥40 mL/min. However, as this medicinal product is known to be substantially excreted by the kidneys, renal function should be more frequently monitored during treatment as these patients may be at greater risk of toxicity.
For additional details about the treatment of patients with renal toxicity, see the SmPC.
Hepatic impairment
Careful consideration of the activity to be administered to patients with hepatic impairment is required since an increased radiation exposure is possible in these patients. The pharmacokinetic profile and safety of LUTATHERA® in patients with baseline severe hepatic impairment (total bilirubin >3 times upper limit of normal, regardless of AST level) have not been studied. Patients with baseline hepatic impairment with either total bilirubin >3 times the upper limit of normal or albuminaemia <30 g/L and INR >1.5 should only be treated with LUTATHERA® after careful benefit-risk assessment. No dose adjustment is recommended for patients with baseline mild or moderate hepatic impairment.
For additional details about the treatment of patients with hepatotoxicity, see the SmPC.
Paediatric population
There is no relevant use of LUTATHERA® in the paediatric population in the indication of treatment of GEP-NETs (excluding neuroblastoma, neuroganglioblastoma and phaeochromocytoma).
Please see the SmPC for full guidance prior to prescribing.
Contraindications to LUTATHERA®¹
Hypersensitivity to the active substance or to any of the excipients listed in section 6.1 of the SmPC
Established or suspected pregnancy or when pregnancy has not been excluded
Kidney failure with creatinine clearance <30 mL/min
Please see the SmPC for full guidance prior to prescribing.
Special warnings and precautions for use¹
Individual benefit-risk justification
For each patient, the radiation exposure must be justifiable by the likely benefit. The activity administered should in every case be as low as reasonably achievable to obtain the required therapeutic effect.
Given the mechanism of action and the tolerance profile of LUTATHERA®, it is not recommended to start treatment with LUTATHERA® in patients with somatostatin receptor-negative or mixed visceral lesions according to somatostatin receptor imaging.
Myelosuppression
Because of the potential for undesirable haematological effects, blood counts must be monitored at baseline and prior to each dose of LUTATHERA® during treatment and until resolution of any eventual toxicity.
Patients with impaired bone marrow function and patients who have received prior chemotherapy or external beam radiotherapy (involving more than 25% of the bone marrow) may be at higher risk of haematological toxicity during LUTATHERA® treatment. Treatment of patients with severely impaired haematological function at baseline and during treatment (e.g., Hb <4.9 mmol/L or 8 g/dL, platelets <75x109/L, or leukocytes <2x109/L) is not recommended unless solely due to lymphopenia.
MDS and acute leukaemia (AL)
Late-onset MDS and AL have been observed after treatment with LUTATHERA®, occurring approximately 29 months (9–45) for MDS and 55 months (32–125) for AL after the first LUTATHERA® infusion. The aetiology of these therapy-related secondary myeloid neoplasms is unclear. Factors such as age >70 years, impaired renal function, baseline cytopenias, prior number of therapies, prior exposure to chemotherapeutic agents (specifically alkylating agents), and prior radiotherapy are suggested as potential risks and/or predictive factors for MDS/AL.
Renal toxicity
Because LUTATHERA® is almost exclusively eliminated through the renal system, it is mandatory to concomitantly administer an amino acid solution containing the amino acids L-lysine and L-arginine. The amino acid solution will help to decrease reabsorption of LUTATHERA® through the proximal tubules, resulting in a significant reduction in the kidney absorbed dose. When the recommended concomitant amino acid solution infusion is delivered over a 4-hour time span, a mean reduction in kidney radiation exposure of about 47% has been reported.
Patients should be encouraged to remain hydrated and to urinate frequently before, on the day of and the day after administration of LUTATHERA® (e.g., 1 glass of water every hour).
Renal function as determined by serum creatinine and calculated creatinine clearance using Cockcroft-Gault formula must be assessed at baseline, during and for at least the first year after treatment.
Patients with renal impairment at baseline or with renal or urinary tract abnormalities, may be at increased risk of toxicity due to increased radiation exposure.
For patients with creatinine clearance <50 mL/min, an increased risk for transient hyperkalaemia due to the amino acid solution should also be taken into consideration (see the Warning and precaution regarding the co-administered renal protective amino acid solution in the SmPC).
Hepatotoxicity
Since many patients referred for LUTATHERA® therapy have hepatic metastasis, it may be common to observe patients with altered baseline liver function. Patients with hepatic metastasis or pre-existing advanced hepatic impairment may be at increased risk of hepatotoxicity due to radiation exposure. Therefore, it is recommended to monitor ALT, AST, bilirubin, serum albumin and INR during treatment.
Hypersensitivity
Cases of hypersensitivity reactions (including isolated angioedema events) have been reported in the post-marketing setting in patients treated with LUTATHERA®. In the event of serious hypersensitivity reactions, the ongoing LUTATHERA® infusion should be discontinued immediately. Appropriate medicinal products and equipment to manage such reactions should be available for immediate use.
Nausea and vomiting
To prevent treatment-related nausea and vomiting, an intravenous bolus of an antiemetic medicinal product should be injected at least 30 minutes prior to the start of amino acid solution infusion to reach the full antiemetic efficacy.
Concomitant use of somatostatin analogues
Somatostatin and its analogues competitively bind to somatostatin receptors and may interfere with the efficacy of LUTATHERA®.
Neuroendocrine hormonal crises
Crises due to excessive release of hormones or bioactive substances may occur following treatment with LUTATHERA®, therefore observation of patients by overnight hospitalisation should be considered in some cases (e.g., patients with poor pharmacological control of symptoms). In case of hormonal crises, recommended treatments are: intravenous high-dose somatostatin analogues, intravenous fluids, corticosteroids, and correction of electrolyte disturbances in patients with diarrhoea and/or vomiting.
Tumour lysis syndrome
Tumour lysis syndrome has been reported following therapy with medicinal products containing lutetium-177. Patients with a history of renal insufficiency and high tumour burden may be at greater risk and should be treated with increased caution. Renal function and electrolyte balance should be assessed at baseline and during treatment.
Radioprotection rules
Patients under treatment with LUTATHERA® should be kept away from others during administration and until the radiation emission limits stipulated by the applicable laws are reached, usually within the 4–5 hours following medicinal product administration. The healthcare professional should determine when the patient can leave the controlled area of the hospital, i.e. when the radiation exposure to third parties does not exceed regulatory thresholds.
Patients should be encouraged to remain hydrated and to urinate frequently before, on the day of and the day after administration of LUTATHERA® (e.g., 1 glass of water every hour) to facilitate elimination. They should also be encouraged to defecate every day and to use a laxative if needed. Urine and faeces should be disposed of according to the national regulations.
Provided the patient’s skin is not contaminated, such as from the leakage of the infusion system or because of urinary incontinence, radioactivity contamination is not expected on the skin and in the vomited mass. However, it is recommended that when conducting standard care or examinations with medical devices or other instruments which come into contact with the skin (e.g., ECG), basic protection measures should be observed such as wearing gloves, installing the material/electrode before the start of radiopharmaceutical infusion, changing the material/electrode after measurement, and eventually monitoring the radioactivity of equipment after use.
Before being discharged, the patient should be instructed in the necessary radioprotection rules for interacting with other members of the same household and the general public, and the general precautions the patient must follow during daily activities after treatment (as given in the next paragraph and the package leaflet) to minimise radiation exposure to others.
After each administration, the following general recommendations can be considered along with national, local and institutional procedures and regulations:
Close contact (less than 1 metre) with other people should be limited for 7 days
For children and/or pregnant women, close contact (less than 1 metre) should be limited to less than 15 minutes per day for 7 days
Patients should sleep in a separate bedroom from other people for 7 days
Patients should sleep in a separate bedroom from children and/or pregnant women for 15 days
Recommended measures in case of extravasation
Disposable waterproof gloves should be worn. The infusion of the medicinal product must be immediately ceased and the administration device (catheter, etc.) removed. The nuclear medicine physician and the radiopharmacist should be informed.
All the administration device materials should be kept in order to measure the residual radioactivity and the activity actually administered and the absorbed dose should be determined. The extravasation area should be delimited with an indelible pen and a picture should be taken if possible. It is also recommended to record the time of extravasation and the estimated volume extravasated.
To continue LUTATHERA® infusion, it is mandatory to use a new catheter, possibly placing it in a contralateral venous access.
No additional medicinal product can be administered to the same side where the extravasation occurred.
In order to accelerate medicinal product dispersion and to prevent its stagnation in tissue, it is recommended to increase blood flow by elevating the affected arm. Depending on the case, aspiration of extravasation fluid, flush injection of sodium chloride 9 mg/mL (0.9%) solution for injection, or application of warm compresses or a heating pad to the infusion site to accelerate vasodilation should be considered.
Symptoms, especially inflammation and/or pain, should be treated. Depending on the situation, the nuclear medicine physician should inform the patient about the risks linked to extravasation injury and give advice about potential treatment and necessary follow-up requirements. The extravasation area must be monitored until the patient is discharged from the hospital. Depending on its severity, this event should be declared as an adverse reaction.
Patients with urinary incontinence
During the first 2 days following administration of this medicinal product, special precautions should be taken with patients with urinary incontinence to avoid spread of radioactive contamination. This includes the handling of any materials possibly contaminated with urine.
Patients with brain metastases
There are no efficacy data in patients with known brain metastases, therefore individual benefit-risk must be assessed in these patients.
Secondary malignant neoplasms
Exposure to ionising radiation is linked with cancer induction and a potential for development of hereditary defects. The radiation dose resulting from therapeutic exposure may result in higher incidence of cancer and mutations. In all cases it is necessary to ensure that the risks of the radiation exposure are less than from the disease itself.
Other patients with risk factors
Patients presenting with any of the conditions below are more prone to develop adverse reactions. Therefore, it is recommended to monitor such patients more frequently during the treatment. Please see Table 3 of the SmPC in case of DMT.
Bone metastasis
Previous oncological radiometabolic therapies with 131I compounds or any other therapy using unshielded radioactive sources
History of other malignant tumours unless the patient is considered to have been in remission for at least 5 years
Contraception in males and females
Female patients of reproductive potential should be advised to use effective contraception during treatment and for 7 months after the last dose of LUTATHERA®.
Male patients with female partners of reproductive potential should be advised to use effective contraception during treatment and for 4 months after the last dose of LUTATHERA®.
Specific warnings and precautions regarding the co-administered renal protective amino acid solution
Hyperkalaemia
A transient increase in serum potassium levels may occur in patients receiving arginine and lysine, usually returning to normal levels within 24 hours from the start of the amino acid solution infusion. Patients with reduced creatinine clearance may be at increased risk for transient hyperkalaemia (see ‘Renal toxicity’ in section 4.4 of the SmPC).
Serum potassium levels must be tested before each administration of amino acid solution. In case of hyperkalaemia, the patient's history of hyperkalaemia and concomitant medicinal product should be checked. Hyperkalaemia must be corrected accordingly before starting the infusion.
In case of pre-existing clinically significant hyperkalaemia, a second monitoring prior to amino acid solution infusion must confirm that hyperkalaemia has been successfully corrected. The patient should be monitored closely for signs and symptoms of hyperkalaemia, e.g. dyspnoea, weakness, numbness, chest pain and cardiac manifestations (conduction abnormalities and cardiac arrhythmias). An ECG should be performed prior to discharging the patient.
Vital signs should be monitored during the infusion regardless of baseline serum potassium levels. Patients should be encouraged to remain hydrated and to urinate frequently before, on the day of and the day after administration (e.g., 1 glass of water every hour) to facilitate elimination of excess serum potassium.
In case hyperkalaemia symptoms develop during amino acid solution infusion, appropriate corrective measures must be taken. In case of severe symptomatic hyperkalaemia, discontinuation of amino acid solution infusion should be considered, taking into consideration the benefit-risk of renal protection versus acute hyperkalaemia.
Heart failure
Due to potential for clinical complications related to volume overload, care should be taken with use of arginine and lysine in patients with severe heart failure defined as class III or class IV in the NYHA classification. Patients with severe heart failure defined as class III or class IV in the NYHA classification should only be treated after careful benefit-risk assessment, taking into consideration the volume and osmolality of the amino acid solution.
Metabolic acidosis
Metabolic acidosis has been observed with complex amino acid solutions administered as part of total parenteral nutrition protocols. Shifts in acid-base balance alter the balance of extracellular-intracellular potassium and the development of acidosis may be associated with rapid increases in plasma potassium.
Specific warnings
Sodium content
This medicinal product contains up to 3.5 mmol (81.1 mg) sodium per vial, equivalent to 4% of the WHO recommended maximum daily intake of 2 g sodium for an adult.
Precautions with respect to environmental hazard see section 6.6 of the SmPC.
Please see the SmPC for full guidance prior to prescribing.
Fertility, pregnancy and lactation¹
Women of childbearing potential
When an administration of radiopharmaceuticals to a woman of childbearing potential is intended, it is important to determine whether or not she is pregnant. Any woman who has missed a period should be assumed to be pregnant until proven otherwise. If in any doubt about her potential pregnancy (if the woman has missed a period, if the period is very irregular, etc.), alternative techniques not using ionising radiation (if there are any) should be offered to the patient. Before the use of LUTATHERA®, pregnancy should be excluded using an adequate/validated test.
Contraception in males and females
LUTATHERA® can cause foetal harm when administered to a pregnant woman.
Female patients of reproductive potential should be advised to use effective contraception during treatment and for 7 months after the last dose of LUTATHERA®.
Male patients with female partners of reproductive potential should be advised to use effective contraception during treatment and for 4 months after the last dose of LUTATHERA®.
Pregnancy
No studies on animal reproductive function have been conducted with LUTATHERA®.
Radionuclide procedures carried out on pregnant women also involve a radiation dose to the foetus. The use of LUTATHERA® is contraindicated during established or suspected pregnancy or when pregnancy has not been excluded due to the risk associated with the ionising radiation. Pregnant women should be advised of the risk to a foetus.
Breast-feeding
It is unknown whether LUTATHERA® is excreted in breast milk. A risk to the breast-fed child associated with ionising radiation cannot be excluded. Breast-feeding should be avoided during treatment with this medicinal product. If treatment with LUTATHERA® during breast-feeding is necessary, the child must be weaned.
Fertility
No animal studies have been performed to determine the effects of LUTATHERA® on male and female fertility. Ionising radiations of LUTATHERA® may potentially have temporary toxic effects on female and male gonads. Genetic consultation is recommended if the patient wishes to have children after treatment. Cryopreservation of sperm or eggs can be discussed as an option for patients before treatment.
Please see the SmPC for full guidance prior to prescribing.
*Cut-off date 18 January 2021. Safety population n=231.2
‡Error bars indicate standard deviations of the mean.2
AE, adverse event; AL, acute leukaemia; ALT, alanin aminotransferase; AST, aspartate aminotransferase; DMT, dose-modifying toxicity; ECG, electrocardiogram; GEP-NET, gastroenteropancreatic neuroendocrine tumour; Hb, haemoglobin; INR, international normalised ratio; LAR, long-acting repeatable; MDS, myelodysplastic syndrome; NYHA, New York Heart Association; RLT, radioligand therapy; SmPC, summary of product characteristics; SOC, standard of care; SSTR, somatostatin receptor; WHO, World Health Organization.
References
LUTATHERA® Summary of Product Characteristics.
Strosberg J, et al. N Engl J Med 2017; 376(2):125–135 and supplementary material.
Strosberg J, et al. Lancet Oncol 2021; 22(12):1752–1763.
Broder MS, et al. World J Gastroenterol 2015;21(6):1945–1955.
UK | January 2026 | FA-11462541-2
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.