Prescribing information (external link)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-2700_0.png.webp?itok=KiWg7nHk)
![Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo. Hero banner. LUTATHERA® (lutetium [177Lu] oxodotreotide) logo.](/uk-en/sites/pro_novartis_com_uk/files/styles/twoup_layout_desktop_1080/public/2025-08/lutathera-home-hero-banner-hcp-1000_0.png.webp?itok=cL3k8yXL)
Mechanism of action
LUTATHERA® (lutetium [177Lu] oxodotreotide) is indicated for the treatment of unresectable or metastatic, progressive, well-differentiated (G1 and G2), somatostatin receptor (SSTR)-positive gastroenteropancreatic neuroendocrine tumours (GEP-NETs) in adults.1
LUTATHERA® Summary of Product Characteristics (SmPC) can be found here
LUTATHERA® delivers SSTR2-targeted radiation directly to overexpressing malignant cells of GEP-NETs1
Based on pre-clinical data.
GEP-NETs strongly overexpress SSTR22
With a high affinity for SSTR2, LUTATHERA® is designed to deliver beta radiation directly to tumour cells, disrupting them from within1
The beta radiation is mostly contained within the tumour, limiting damage to neighbouring healthy cells1
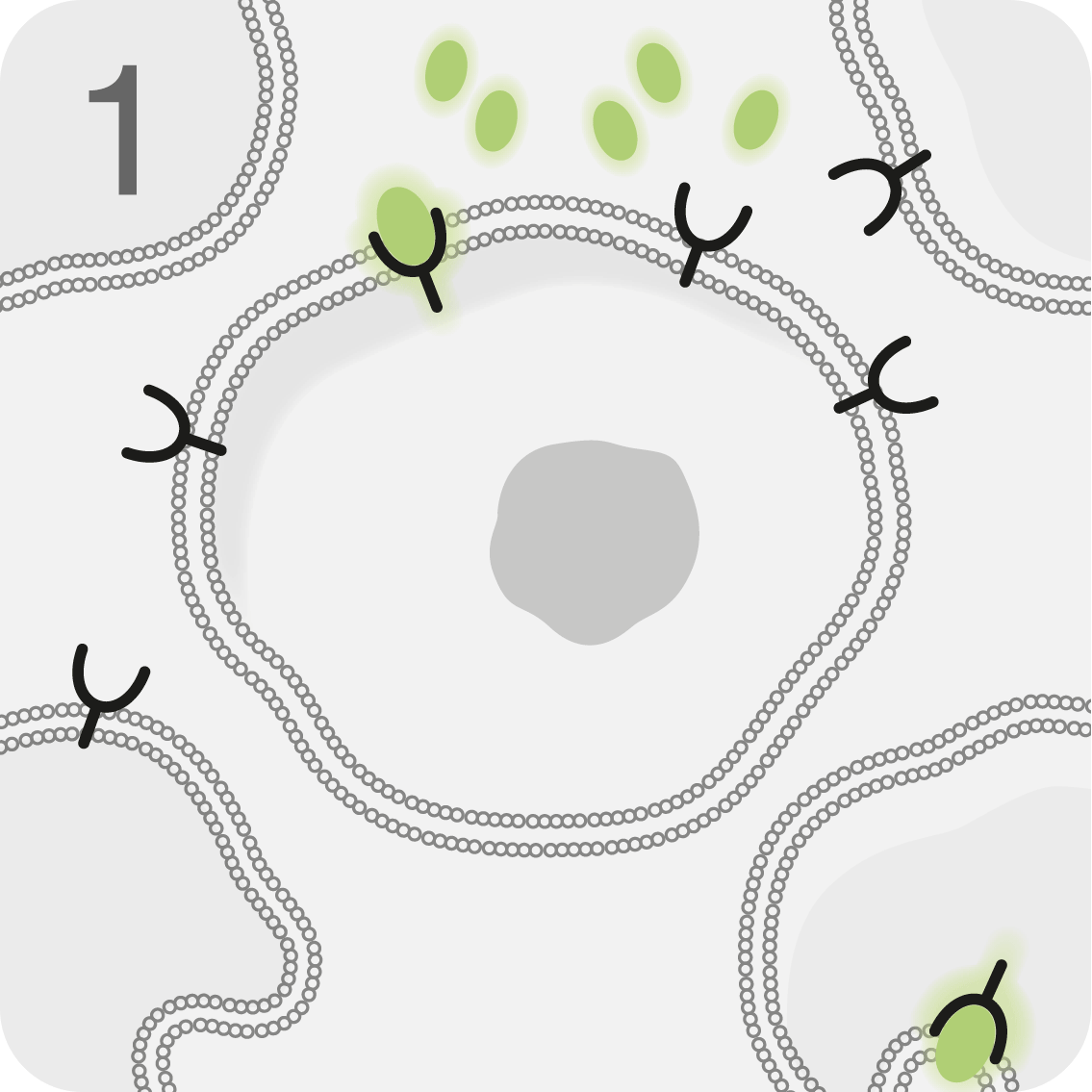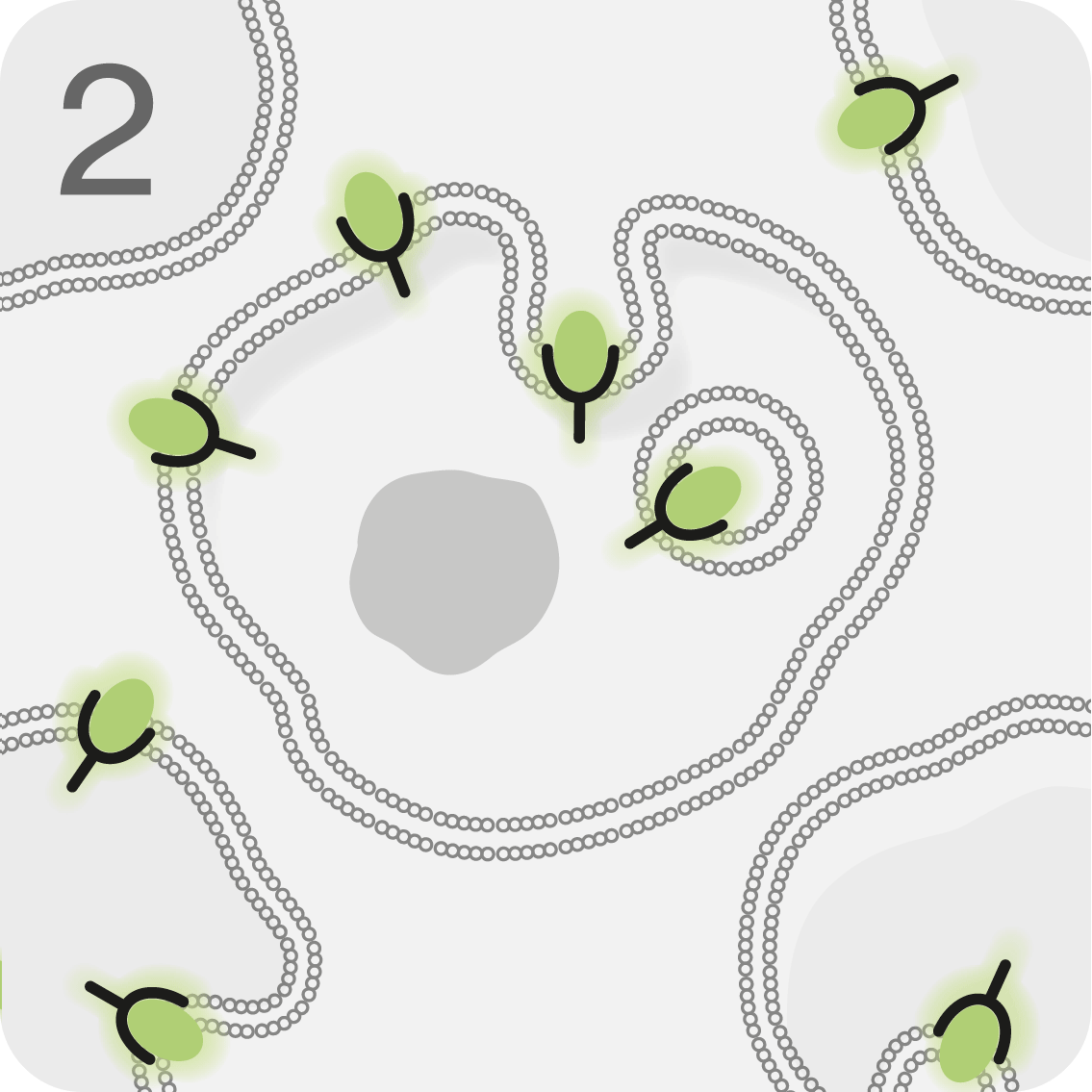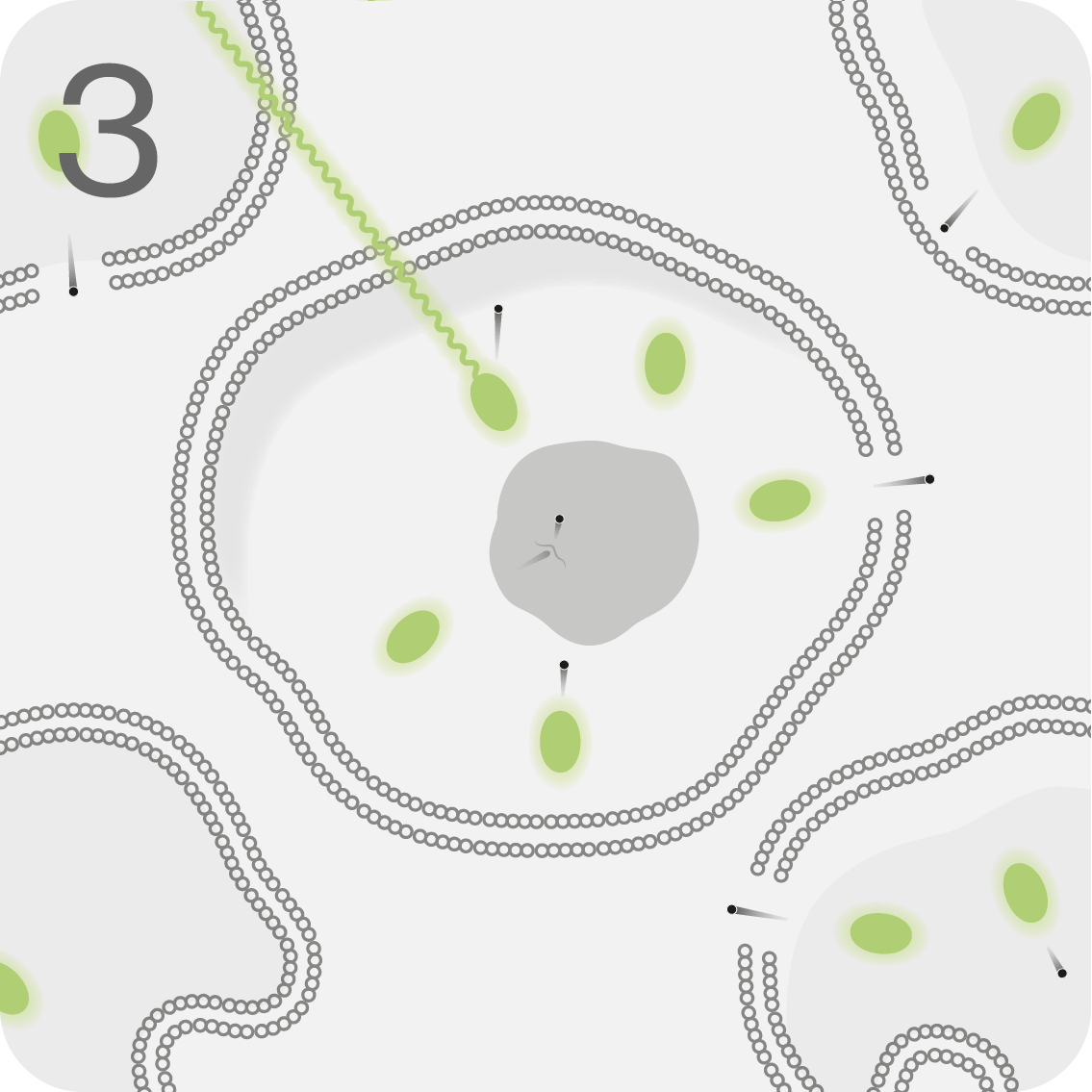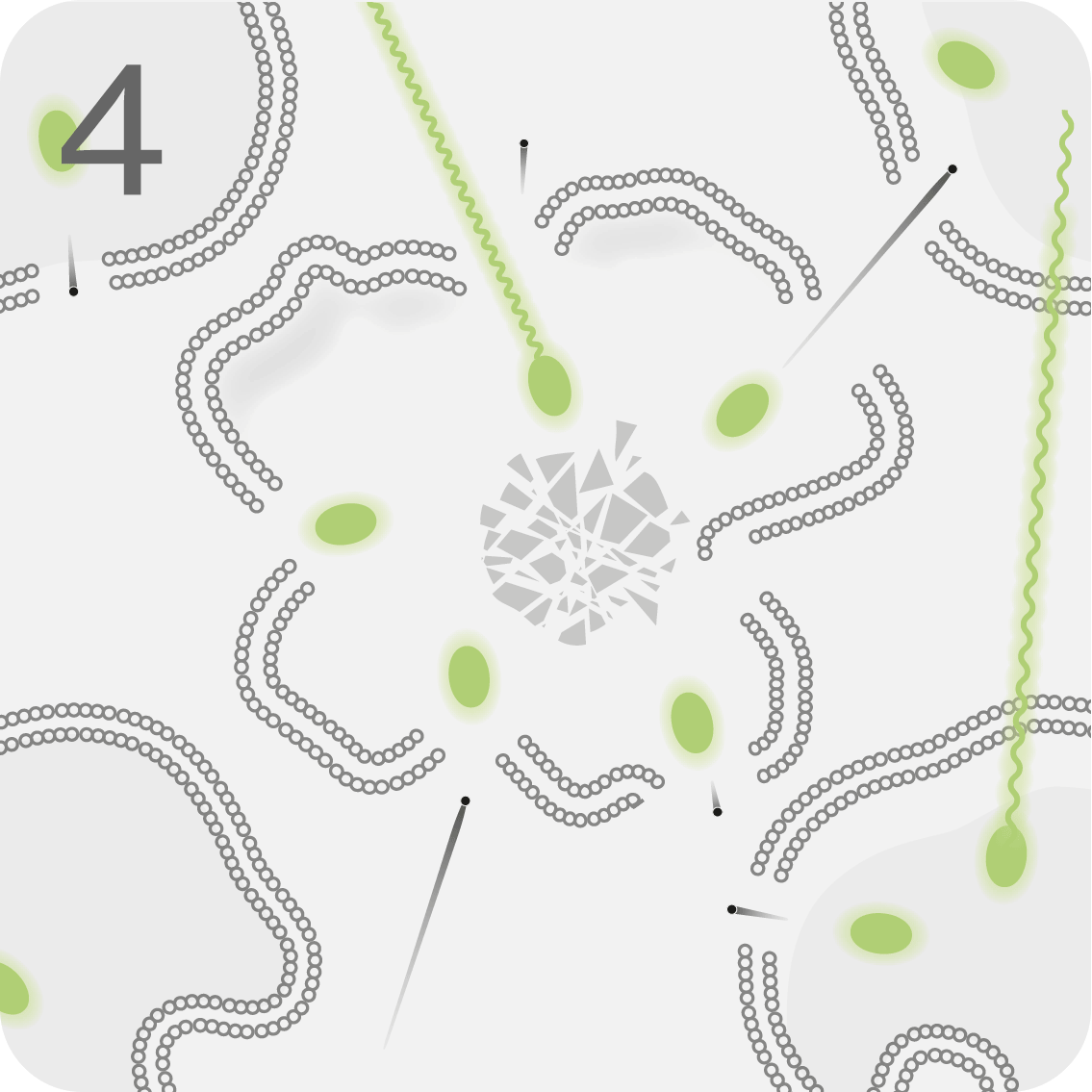
Stage 1
LUTATHERA® is infused into the bloodstream, where it targets and binds to malignant cells overexpressing SSTR2.1
Stage 2
LUTATHERA® is then internalised into the cell.3
Stage 3
In the cell, it delivers beta radiation, with an average tissue penetration of 0.67mm.1
Stage 4
The radiation disrupts the targeted tumour cell from within, with a limited effect on neighbouring normal cells.1
GEP-NET, gastroenteropancreatic neuroendocrine tumour; RLT, radioligand therapy; SmPC, summary of product characteristics; SSTR, somatostatin receptor.
References
LUTATHERA® Summary of Product Characteristics.
Thundimadathil J. J Amino Acids 2012;2012:967347.
Hennrich U & Kopka K. Pharmaceuticals (Basel) 2019;12(3):114.
UK | December 2025 | FA-11462531-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.





