Need more information?
Our dedicated team of Novartis representatives based throughout the UK are on hand to answer your local queries.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
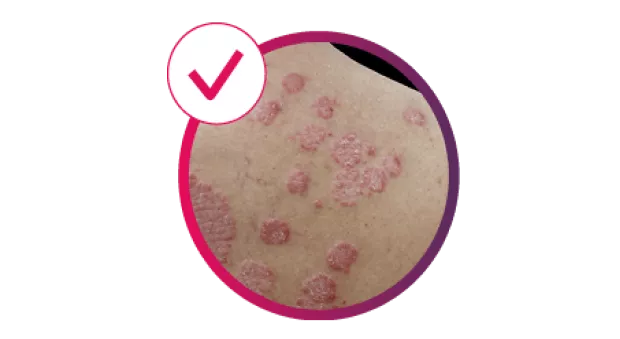
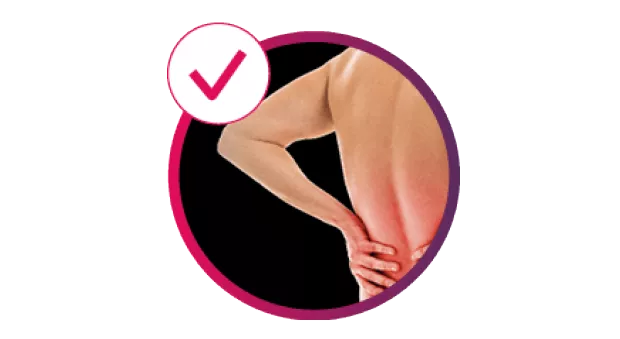
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy.1
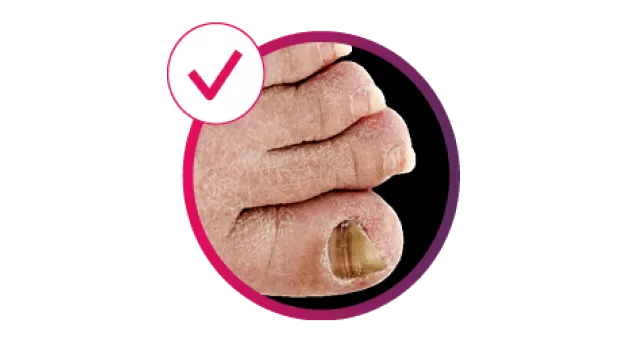
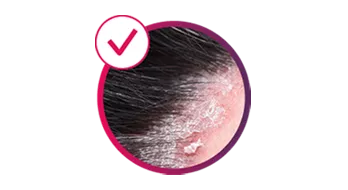
Cosentyx treats the visible signs of PsO, delivering efficacy in skin with scalp and nail involvement, and helps reduce the risk of irreversible joint damage progression.2–5
Observed and NRI endpoints; not statistically tested.
sustained PASI 100 at Year 1
(observed; n=41)¶2
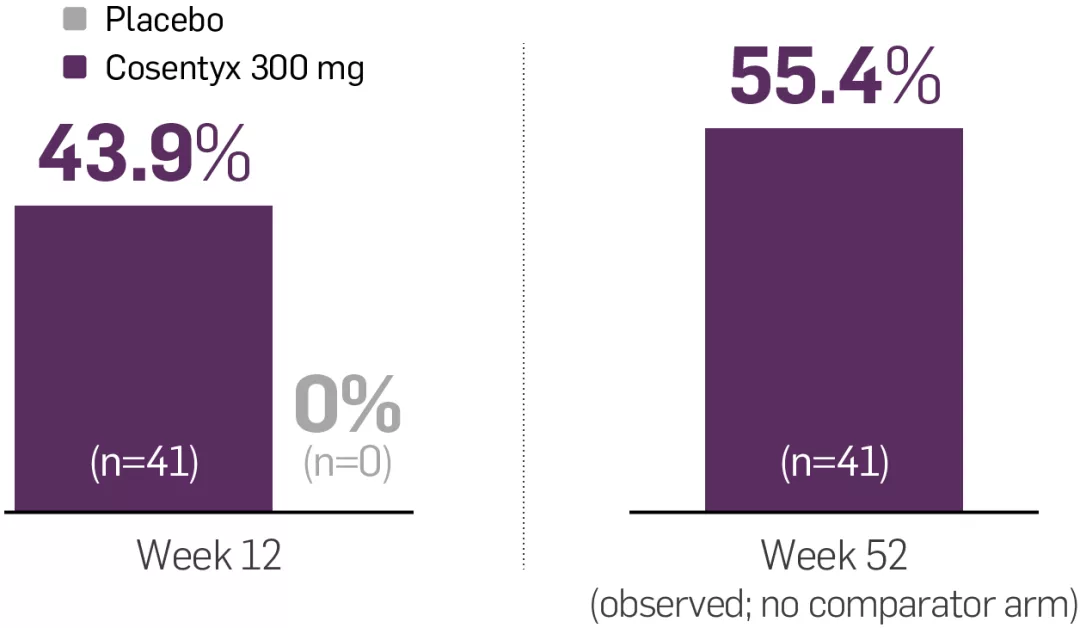
MATURE: co-primary endpoints of PASI 75 and IGA mod 2011 0/1 at Week 12 for Cosentyx Al 300 mg vs placebo were met (95% vs 10% and 76% vs 8%, respectively; p<0.0001)2
achieved ACR50 at Week 24
(NRI; n=81)‡8
ULTIMATE: primary endpoint of GLOESS score for Cosentyx vs placebo at Week 12 was met (−9 vs −6, respectively; p=0.004)9
sustained complete resolution at Year 5
(observed; n=51)§10
FUTURE 2: primary endpoint of ACR20 response at Week 24 for Cosentyx 300 mg or 150 mg vs placebo was met (54% in 300 mg; 51% in 150 mg; 15% in placebo; p<0.0001)11
sustained reduction in mNAPSI at 2.5 years
(observed; n=66)†12
TRANSFIGURE: primary endpoint of percentage change from baseline in mean NAPSI score at Week 16 for Cosentyx 300 mg or 150 mg vs placebo was met (−45% for 300 mg, −38% for 150 mg vs −11% for placebo, p<0.001)5
sustained ASAS40 at Year 1
(observed; n=139)‖6
MAXIMISE: primary endpoint of ASAS20 response at Week 12 for Cosentyx 300 mg vs placebo was met (63% vs 31%, respectively; p<0.0001)6
sustained complete resolution at Year 5 (observed; n=40)§10
FUTURE 2: primary endpoint of ACR20 response at Week 24 for Cosentyx 300 mg or 150 mg vs placebo was met (54% in 300 mg; 51% in 150 mg; 15% in placebo; p<0.0001)11
sustained PSSI 90 clearance at Week 12
(NRI; n=51)**4
SCALP: primary endpoint of PSSI 90 response at Week 12 for Cosentyx 300 mg vs placebo was met (53% vs 2.0%, respectively; p<0.001)4
FAST = efficacy at 12 weeks2
LASTING = efficacy at 52 weeks2
PASI 100 skin clearance rates over time in biologic-naïve and -experienced patients2
p<0.0001 vs placebo at Week 12.
Adapted from Sigurgeirsson B, et al. 2022.2
~6 out of 10 patients taking Cosentyx 300 mg achieved PASI 100 at 1 year2
PASI 75 response rates with Cosentyx via the UnoReady® 300 mg pen were superior to placebo at Week 12 (co-primary endpoint; 95.1% vs 10%, respectively, p<0.0001). IGA mod 2011 0/1 response rates with Cosentyx via the UnoReady® 300 mg pen were also superior to placebo at Week 12 (co-primary endpoint; 75.6% vs 7.6%, respectively, p<0.0001).2
MATURE was a 52-week, multicentre, randomised, double-blind, placebo-controlled Phase III trial that evaluated the efficacy, safety, tolerability and pharmacokinetics (PK) of Cosentyx administered to patients with moderate to severe PsO via one 300 mg/2 mL UnoReady® pen injection (n=41) or two 150 mg/1 mL pre-filled syringe injections (n=41) vs placebo (n=40). Patients self-administered treatment at Weeks 0, 1, 2, 3, 4 and 8, followed by dosing every 4 weeks starting at Week 12 up to Week 48. The patients in the placebo group who did not achieve PASI 90 were administered Cosentyx after Week 12. The co-primary endpoints were PASI 75 and IGA mod 2011 0/1 response rates at Week 12. The key secondary endpoint was PASI 90 response rate at Week 12. Other secondary endpoints were evaluation of PK data and efficacy of Cosentyx at Week 52.2
Observed responses after Cosentyx treatment, based on PASI 90 and PASI 100, were sustained through the 5-year follow-up period.13
Adapted from Augustin M, et al. 2024.13
Observational data, no statistical testing was performed.
SERENA was a non-interventional, prospective study. The primary objective of this 5-year analysis was to assess long-term retention of Cosentyx in patients with PsO, PsA and ankylosing spondylitis (AS).14 1740 patients with moderate to severe PsO were included in this study (target population set). 913 (52.5%) patients did not complete the study; the most common reasons for discontinuation included lack of efficacy (25.4%), patient decision (7.6%), physician decision (5.5%), lost to follow-up (4.8%) and adverse event (4.7%).13
SERENA was a non-interventional, prospective study conducted across 18 primarily European countries that assessed the retention and effectiveness of Cosentyx up to 5 years in patients with moderate to severe chronic PsO, active PsA, or AS. Patients received ≥16 weeks of Cosentyx treatment before enrolment. Retention rate was derived from Kaplan–Meier estimates. Effectiveness assessments included swollen joint count and tender joint count in patients with PsA up to 5 years.13,14
Artist’s representation of early radiographic damage. These images do not depict real patients. For illustrative purposes only.
FUTURE 5 analysis through 2 years. The primary endpoint of proportion of patients achieving ACR20 response at Week 16 was met (Cosentyx 300 mg 62.6% vs placebo 27.4%, p<0.0001). The study included 996 patients with active PsA, 222 of whom were taking Cosentyx 300 mg. No structural progression responders are those subjects who have a change in van der Heijde modified total Sharp score relative to baseline of ≤0.5 during the analysis period.15
Rheumatological clinical societies and associations recommend IL-17 inhibitors:
✔ GRAPPA 2021 guidance strongly recommends an IL-17 inhibitor as a first-line biologic following inadequate response to DMARDs, across all 6 key manifestations of PsA16
✔ IL-17 inhibitors are recommended by BSR as a first-line biologic option for key manifestations of PsA17
Please note Cosentyx is recommended for patients with PsA when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. Please see the SmPC for full information.1
✔ BAD recommends an IL-17 inhibitor as a first-line biologic for adult patients with PsO and PsA18
Cosentyx is only licensed in adults with PsA (alone or in combination with MTX) if there is an inadequate response to a previous DMARD.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*The 6 key manifestations of PsA are joints, axial, skin, enthesitis, dactylitis, and nails.6
†TRANSFIGURE: the primary endpoint of NAPSI improvement score vs placebo at Week 16 was met (−45.3% for Cosentyx 300 mg vs −10.8% for placebo, p<0.0001).5 Observed data in patients with moderate to severe nail PsO in the 300 mg treatment group (n=66); in the respective 150 mg treatment group (n=67), there was a mean NAPSI improvement of −63.6% sustained at Year 2.5.12
‡ULTIMATE: nonresponder imputation data in biologic-naïve patients originally randomly assigned to Cosentyx.9 The primary endpoint of GLOESS score vs placebo at Week 12 was met (−9% for Cosentyx 300 mg vs −6% for placebo, p=0.004).9
§FUTURE 2: observed data for the 300 mg treatment group of biologic-naïve patients with this symptom at baseline; 82% in the respective 150 mg group maintained complete resolution of dactylitis through Year 5 (n=28); 75% in the respective 150 mg group maintained complete resolution of enthesitis through Year 5 (n=64). The primary endpoint of ACR20 vs placebo at Week 24 was met (54% for Cosentyx 300 mg vs 15.3% with placebo, p<0.0001).10,11
¶MATURE: a 52-week, multicentre, randomised, double-blind, placebo-controlled Phase III trial (n=122). Co-primary endpoints were PASI 75 and IGA 0/1 response rates at Week 12 vs placebo. PASI 75 was met (95.1% for Cosentyx 300 mg vs 10% for placebo, p<0.0001). IGA mod 2011 0/1 was also met (75.6% for Cosentyx 300 mg vs 7.6% for placebo, p<0.0001).2
‖MAXIMISE: observed data in biologic-naïve patients in the 300 mg treatment group (n=139); in the respective 150 mg treatment group, 65% achieved ASAS40 at Year 1 (n=141). The primary endpoint of ASAS20 vs placebo at Week 12 was met (63% for Cosentyx 300 mg vs 31% for placebo, p<0.0001).6
**SCALP: a 24-week randomised, double-blind, placebo-controlled, parallel-group, multicentre, Phase IIIb study (n=102). Patients with moderate to severe scalp PsO received Cosentyx 300 mg or placebo at baseline, Weeks 1, 2 and 3, and then every 4 weeks from Weeks 4 to 20. The final efficacy and safety evaluations were performed at Week 24. The primary endpoint of PSSI 90 vs placebo at Week 12 was met (52.9% for Cosentyx 300 mg vs 2% for placebo, p<0.001).4
††Since first indication in eligible adults with moderate to severe PsO.19
ACR, American College of Rheumatology; AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis International Society; BAD, British Association of Dermatologists; BSR, British Society for Rheumatology; DMARD, disease-modifying anti-rheumatic drug; ERA, enthesitis-related arthritis; GLOESS, Global OMERACT/EULAR ultrasound synovitis score; GRAPPA, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis; HCP, healthcare professional; HS, hidradenitis suppurativa; IGA, investigator’s global assessment; IL, interleukin; JPsA, juvenile psoriatic arthritis; MDA, minimal disease activity; mNAPSI, mean nail psoriasis severity index; MTX, methotrexate; NAPSI, nail psoriasis severity index; nr-axSpA, non-radiographic axial spondyloarthritis; NRI, non-responder imputation; PASI, psoriasis area and severity index; PK, pharmacokinetics; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSSI, psoriasis scalp severity index; QoL, quality of life; SmPC, summary of product characteristics.
References
Cosentyx (secukinumab) Summary of Product Characteristics.
Sigurgeirsson B, et al. Dermatol Ther 2022;35(3):e15285.
Mease PJ, et al. RMD Open 2021;7(2):e001600.
Bagel J, et al. J Am Acad Dermatol 2017;77(4):667–674.
Reich K, et al. Br J Dermatol 2019;181(5):954–966.
Baraliakos X, et al. Ann Rheum Dis 2021;80(5):582–590.
Nash P, et al. Clin Exp Rheumatol 2022;40(5):952–959.
Boers M, et al. Poster POS0917. EULAR European Congress of Rheumatology. 2–5 June 2021, Virtual Congress.
D’Agostino MA, et al. Rheumatology 2022;61(5):1867–1876.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235.
McInnes IB, et al. Lancet 2015;386(9999):1137–146.
Reich K, et el. Br J Dermatol 2021;184(3):425–436.
Augustin M, et al. Poster P3355. European Academy of Dermatology and Venereology (EADV) Congress. 25–28 September 2024, Amsterdam, The Netherlands.
Kiltz U, et al. Adv Ther 2020;37:2865–2883.
Mease P, et al. Ann Rheum Dis 2018;77(6):890–897.
Coates LC, et al. Nat Rev Rheumatol 2022;18(8):465–479.
Tucker L, et al. Rheumatology 2022(9);61:e255–e266.
Smith CH, et al. Br J Dermatol 2020(4);183:628–637.
European Medicines Agency. Assessment report: Cosentyx (EMA/CHMP/389874/2014). Available from: https://www.ema.europa.eu/en/documents/assessment-report/cosentyx-epar-public-assessment-report_en.pdf [Accessed July 2025].
UK | July 2025 | FA-11386224-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.