Need more information?
Our dedicated team of Novartis representatives based throughout the UK are on hand to answer your local queries.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated.1
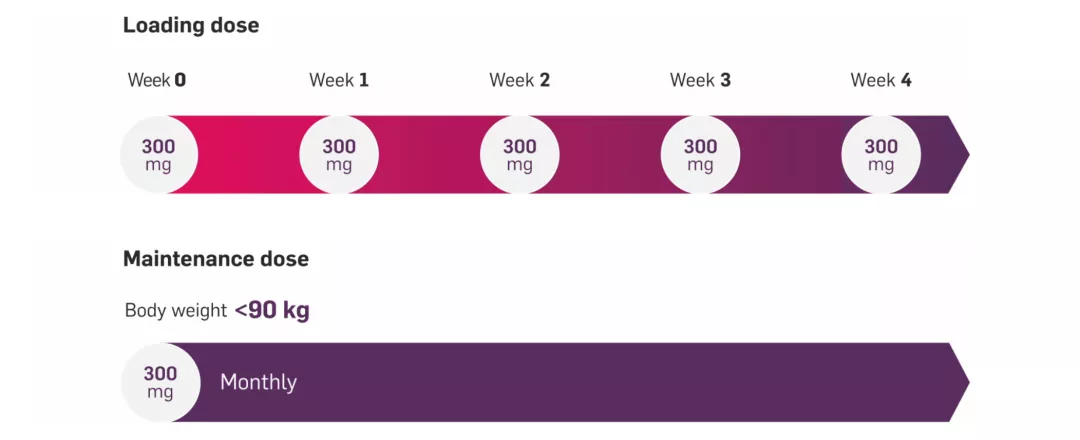
The recommended dose of Cosentyx in adults with PsO is 300 mg delivered at Weeks 0, 1, 2, 3 and 4, followed by a monthly maintenance dose.1
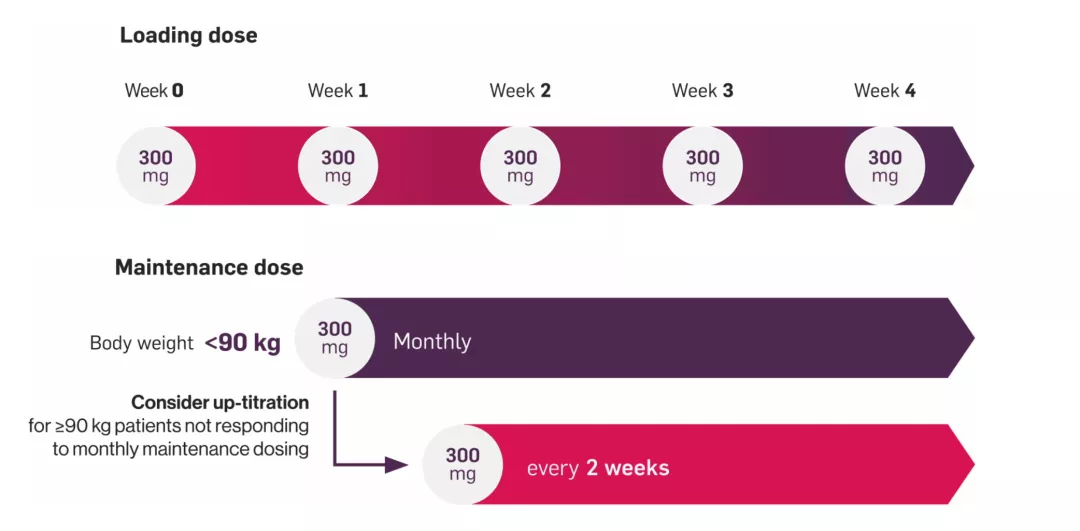
Based on clinical response, patients with PsO weighing ≥90 kg may benefit from a maintenance dose of 300 mg every 2 weeks.1 Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.
For patients who weigh <90 kg, the recommended Cosentyx dose is 300 mg with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
For patients who weigh ≥90 kg, the recommended Cosentyx dose is 300 mg at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Maintenance dosing may be up-titrated for patients weighing ≥90 kg to 300 mg every 2 weeks depending on clinical response.1
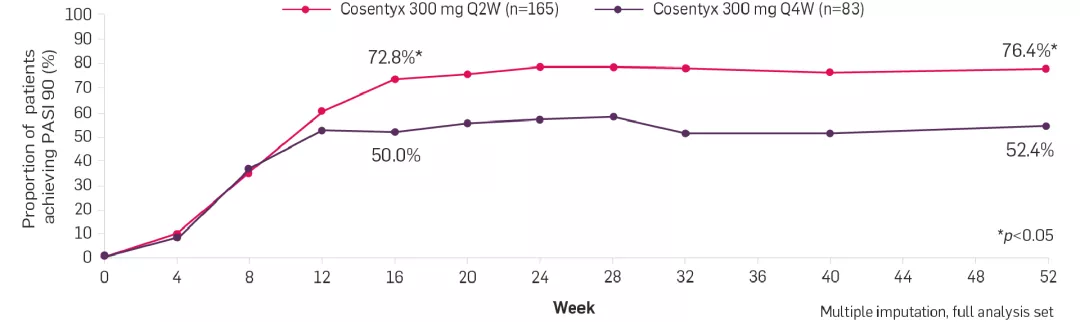
You can tailor the dosing of Cosentyx based on the clinical response of your patients with PsO weighing ≥90 kg. In the A2324 Q2W clinical trial, FAST (Week 16) and observed LONG-LASTING (Week 52) skin clearance was seen in patients (≥90 kg) treated with Cosentyx 300 mg Q2W vs 300 mg Q4W.2
Primary endpoint of PASI 90 response at Week 16 for Cosentyx 300 mg Q2W vs Q4W was met (73.2% vs 55.5%; one-sided p=0.0003).2
Please note the p value of p=0.0003 is related to the primary endpoint. For the exploratory endpoint at Week 52, the p value is <0.05.
Adapted from Augustin M, et al. 2022.2
A2324 Q2W was a multicentre, double-blind, parallel-group trial on patients with moderate to severe PsO weighing ≥90 kg (N=331) treated with Cosentyx 300 mg Q2W or Q4W.
75 mg pre-filled syringe
Cosentyx offers a range of pre-filled injections. The pre-filled syringes are available in three dosages: 75 mg, 150 mg, and 300 mg; the pre-filled pens are available in two dosages: 150 mg and 300 mg. The 150 mg and 300 mg solution for injection in pre-filled syringe and in pre-filled pen are not indicated for administration to paediatric patients with a weight <50 kg.1
*MATURE was a 52-week, randomised, double-blind, placebo controlled study that evaluated efficacy, safety, tolerability and PK of Cosentyx in patients with moderate to severe PsO via one 300 mg/2 ml UnoReady pen injection (n=41) or two 150 mg/1 ml pre-filled syringe injections (n=41) vs placebo. Patients self-administered treatment at Weeks 0, 1, 2, 3, 4 and 8 followed by Q4W dosing starting at Week 12 up to Week 48. The co-primary endpoints of PASI 75 and IGA mod 2011 0/1 response rates at Week 12 were met (p<0.0001 for both).3
The Cosentyx UnoReady® pen delivers the potential benefits of Cosentyx 300 mg in one injection. In the MATURE study at 28 weeks 100% of patients (n=37) were ‘satisfied’ or ‘very satisfied’ with using the UnoReady® pen.1,3,4
There are some differences between using the Cosentyx SensoReady® and UnoReady® pen that eligible patients should be aware of:†4,5
†After proper training in subcutaneous injection technique, patients may self-inject Cosentyx or may be injected by a caregiver if a physician determines that this is appropriate. However, the physician should ensure appropriate follow-up of patients. Patients or caregivers should be instructed to inject the full amount of Cosentyx according to the instructions provided in the package leaflet. Comprehensive instructions for administration are given in the package leaflet.1
In paediatric PsO treatment, the recommended Cosentyx dose varies depending on the child’s body weight.1
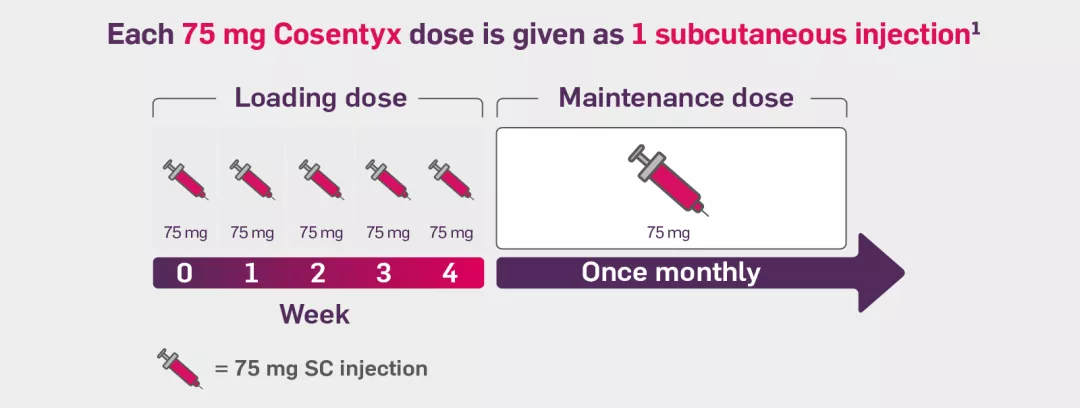
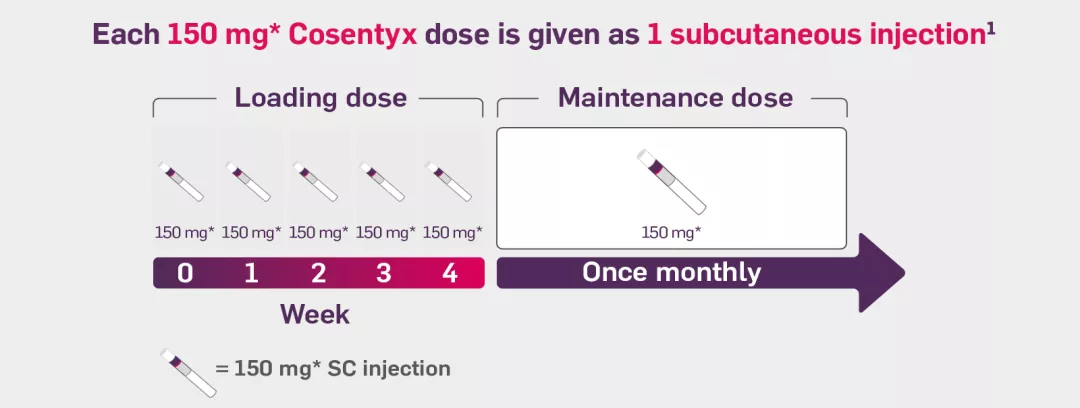
The dosing schedule of Cosentyx begins with the initial dose at Weeks 0, 1, 2, 3 and 4, followed by a monthly maintenance dose. Each dose of Cosentyx, whether 75 mg, 150 mg, or 300 mg, is administered as a single subcutaneous injection. The 300 mg dose may be given as one 300 mg injection or two 150 mg injections.1
If your patient weighs under 50 kg, the dosing regimen for Cosentyx involves a weekly 75 mg subcutaneous injection during the initial 4-week phase, followed by a monthly maintenance dose of 75 mg. The Cosentyx injection doses are administered as a single 75 mg subcutaneous injection.1
The 150 mg and 300 mg solution for injection, available in pre-filled syringe and pen formats, is not indicated for paediatric patients with a weight below 50 kg. Cosentyx may be available in other strengths and/or presentations depending on the individual treatment needs.1
If your patient weighs over 50 kg, the recommended Cosentyx dosing schedule begins with a weekly subcutaneous injection of 150 mg during the initial dosing phase, followed by a monthly maintenance dose of 150 mg. This dose can be increased to 300 mg if deemed beneficial for certain patients. The Cosentyx 300 mg dose can be administered as a single subcutaneous injection or as two separate injections of 150 mg each.1
Cosentyx may be available in other strengths and/or presentations depending on the individual treatment needs.1
The efficacy of Cosentyx across its various indications has been established through clinical studies. Based on the available evidence, a clinical response is normally achieved within the first 16 weeks of treatment, however, if your patients don’t demonstrate a response to the prescribed Cosentyx dose within this timeframe, consideration should be given to discontinuing the treatment. Some patients with an initial partial response may subsequently improve with continued treatment beyond 16 weeks.1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated.1
This is not an exhaustive list of warnings and precautions. Please refer to the Cosentyx SmPC for full information.1
The most frequently reported adverse reactions are upper respiratory tract infections (17.1%) (most frequently nasopharyngitis, rhinitis).1
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated. Please refer to the Cosentyx SmPC for full product information and administration, including dosing in special populations, before prescribing.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
AS, ankylosing spondylitis; ERA, enthesitis-related arthritis; HBV, hepatitis B virus; HS, hidradenitis suppurativa; IGA mod 2011/01, Investigator's Global Assessment modified 2011 Investigator's Global Assessment modified 2011; JPsA, juvenile psoriatic arthritis; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PASI, psoriasis area and severity index; PK, pharmacokinetics; PsA, psoriatic arthritis; PsO, plaque psoriasis; Q2W, every 2 weeks; Q4W, every 4 weeks; SmPC, Summary of Product Characteristics.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Augustin M, et al. Br J Dermatol 2022;186:942-954.
Sigurgeirsson B, et al. Dermatol Ther 2022;35(3):e15285.
Cosentyx 300 mg solution for injection pre-filled pen. Patient Information Leaflet.
Cosentyx 150 mg solution for injection pre-filled pen. Patient Information Leaflet.
UK | September 2025 | FA-11448996-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.