Successive time periods of PSUR shown with cumulative rate: 26 Dec 2014 to 25 June 2015; 26 June 2015 to 25 Dec 2015; 26 Dec 2015 to 25 June 2016; 26 June 2016 to 25 Dec 2016; 26 Dec 2016 to 25 Dec 2017; 26 Dec 2017 to 25 Dec 2018; 26 Dec 2018 to 25 Dec 2019; 26 Dec 2019 to 25 Dec 2020; 26 Dec 2020 to 25 Dec 2023.5
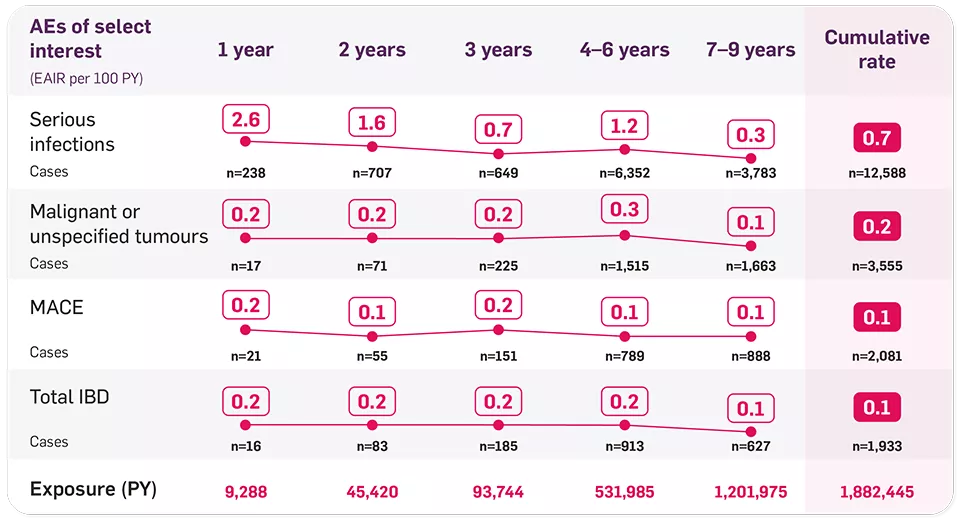
The most frequently reported adverse reactions are upper respiratory tract infections (17.1%) (most frequently nasopharyngitis, rhinitis).1
Caution should be exercised when considering the use of Cosentyx in patients with a chronic infection or a history of recurrent infection. If a patient develops a serious infection, the patient should be closely monitored and Cosentyx should not be administered until the infection resolves.1
Patients should be evaluated for tuberculosis infection prior to initiating treatment with Cosentyx. Cosentyx should not be given to patients with active tuberculosis. In patients with latent tuberculosis, anti‑tuberculosis therapy should be considered prior to initiation of Cosentyx.1
Cosentyx is not recommended in patients with IBD. If a patient develops signs and symptoms of IBD or experiences an exacerbation of pre-existing inflammatory bowel disease, Cosentyx should be discontinued and appropriate medical management should be initiated.1
Please refer to the SmPC for full safety information before prescribing.1