Prescribing information (external link)


Patient management and psoriatic disease
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy.1
Overall management of psoriatic disease requires a holistic approach
Psoriatic disease consists of three major domains: skin inflammation, joint involvement (PsA) and vascular inflammation.2 Psoriatic disease is associated with a number of comorbidities and can be strongly influenced by risk and trigger factors.3 Comorbidities and the presence of PsA are important denominators for drug selection.3
Does your treatment choice address the holistic needs of PsO?
Spheres of psoriatic disease2
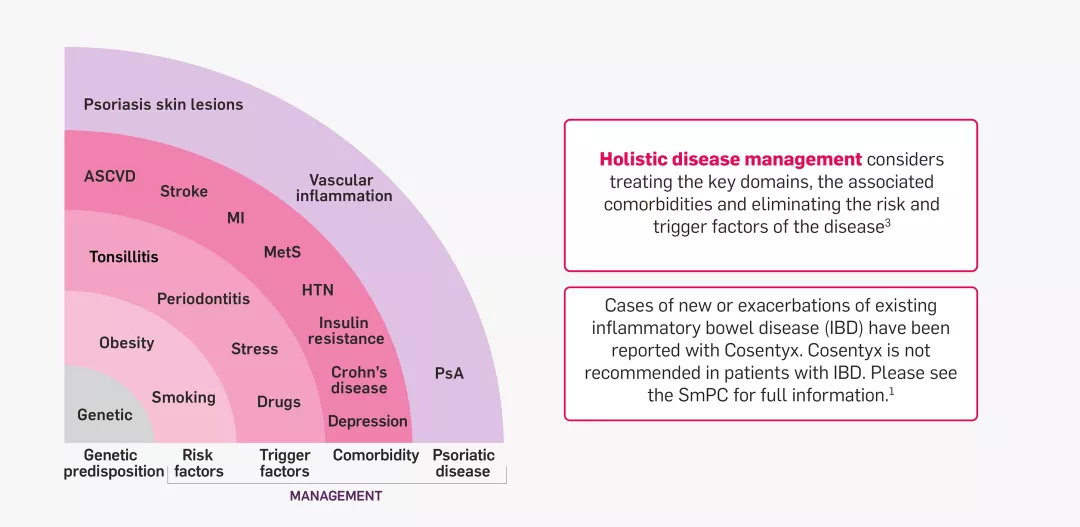
Adapted from Mrowietz U, et al. 2023.2
Incidence of clinical manifestations in patients with PsO4–7
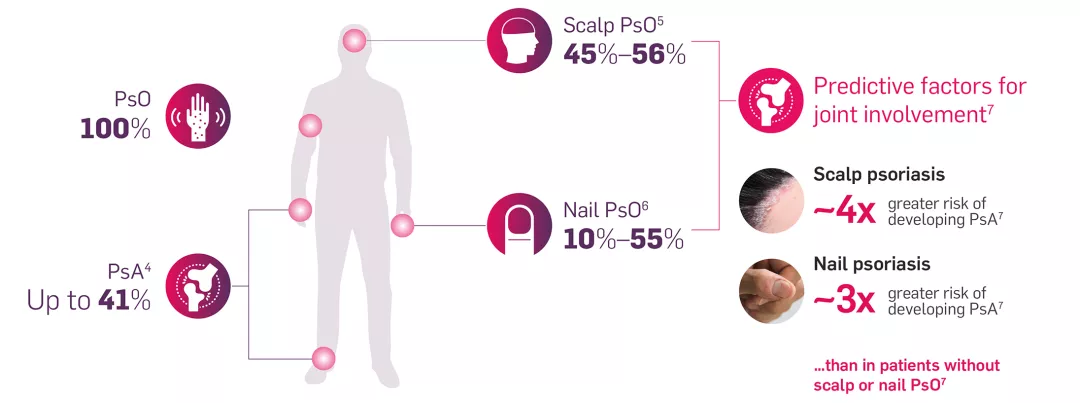
Identifying risk factors in patients with PsO is important to help delay the development of PsA joint damage8,9

These are representative patient images.
Find out more about the efficacy of Cosentyx in your eligible patients with PsO and PsA
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*Patients with newly diagnosed, treatment-naïve PsA fulfilling the ClASsification for Psoriatic Arthritis (CASPAR) classification criteria of ≤5 years symptom duration (N=122) were recruited as part of the Leeds Spondyloarthropathy Register for Research and Observation and underwent conventional radiography and ultrasound examination of hands and feet.10
AS, ankylosing spondylitis; ASCVD, atherosclerotic cardiovascular disease; CASPAR, ClASsification for Psoriatic Arthritis; ERA, enthesitis-related arthritis; HS, hidradenitis suppurativa; HTN, hypertension; IBD, inflammatory bowel disease; JPsA, juvenile psoriatic arthritis; MetS, metabolic syndrome; MI, myocardial infarction; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Mrowietz U, et al. J Eur Acad Dermatol Venereol 2023;37(9):1731–1738.
Mrowietz U, et al. Exp Dermatol 2014;23(10):705–709.
Singh JA, et al. Arthritis Rheumatol 2019;71(1):5–32.
Dopytalska K, et al. Rheumatologia 2018;56(6):392–398.
Oram Y, et al. Dermatol Res Pract 2013;2013:180496.
Wilson FC, et al. Arthritis Rheum 2009;61(2):233–239.
Patt YS, et al. Clin Exp Rheumatol 2024;42:1856–1866.
Haroon M, et al. Ann Rheum Dis 2015;74(6):1045–1050.
Hen O, et al. RMD Open 2024;10(2):e003841.
UK | December 2025 | FA-11433155-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

