

Cosentyx® (secukinumab) and PsO with PsA efficacy
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy.1
Explore Cosentyx efficacy in PsO with PsA
Download a summary of the key Cosentyx efficacy data here
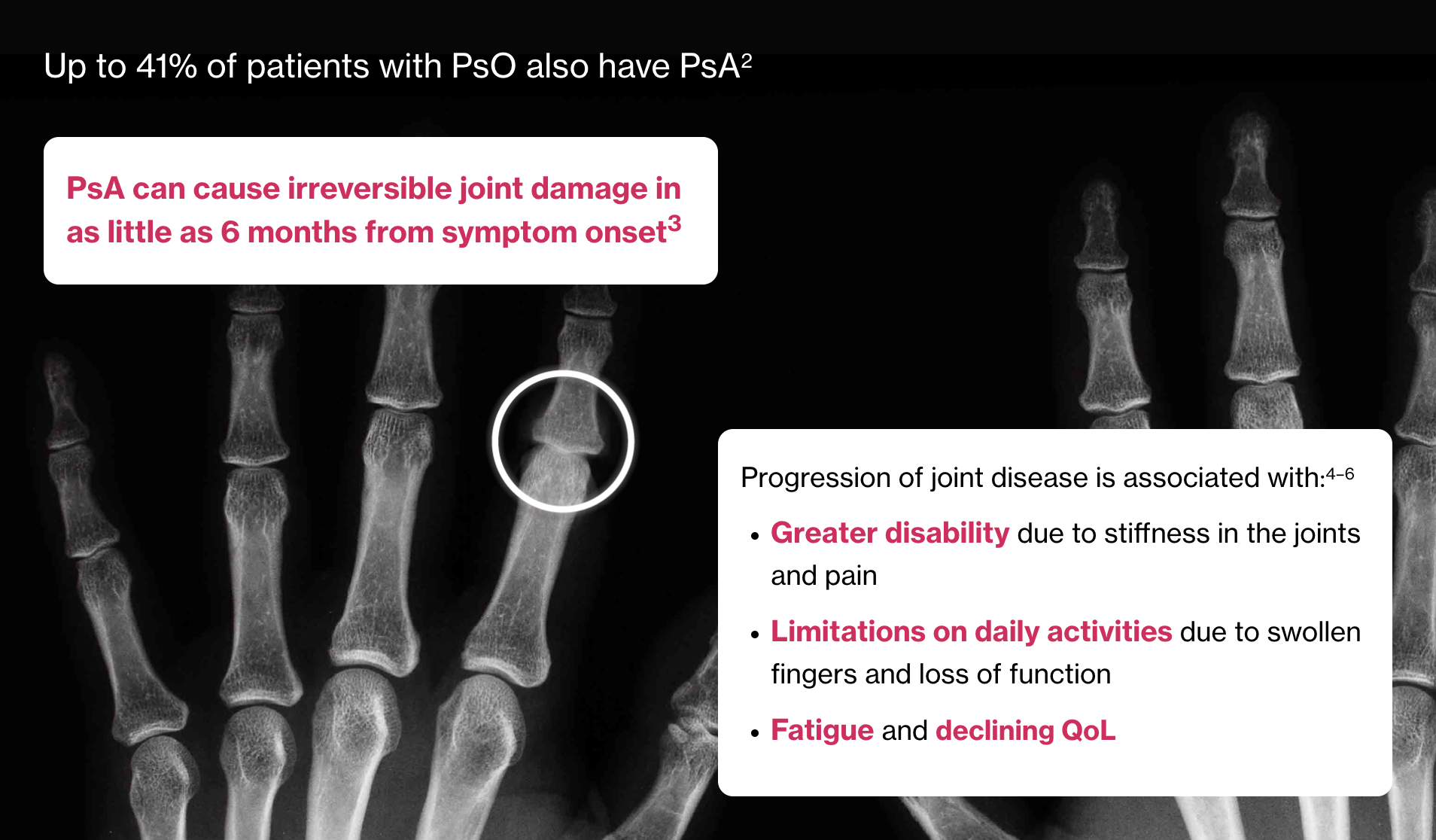
Artist’s representation of early radiographic damage.
Identifying PsA risk factors in your PsO patients
Red flags indicating PsA risk include:
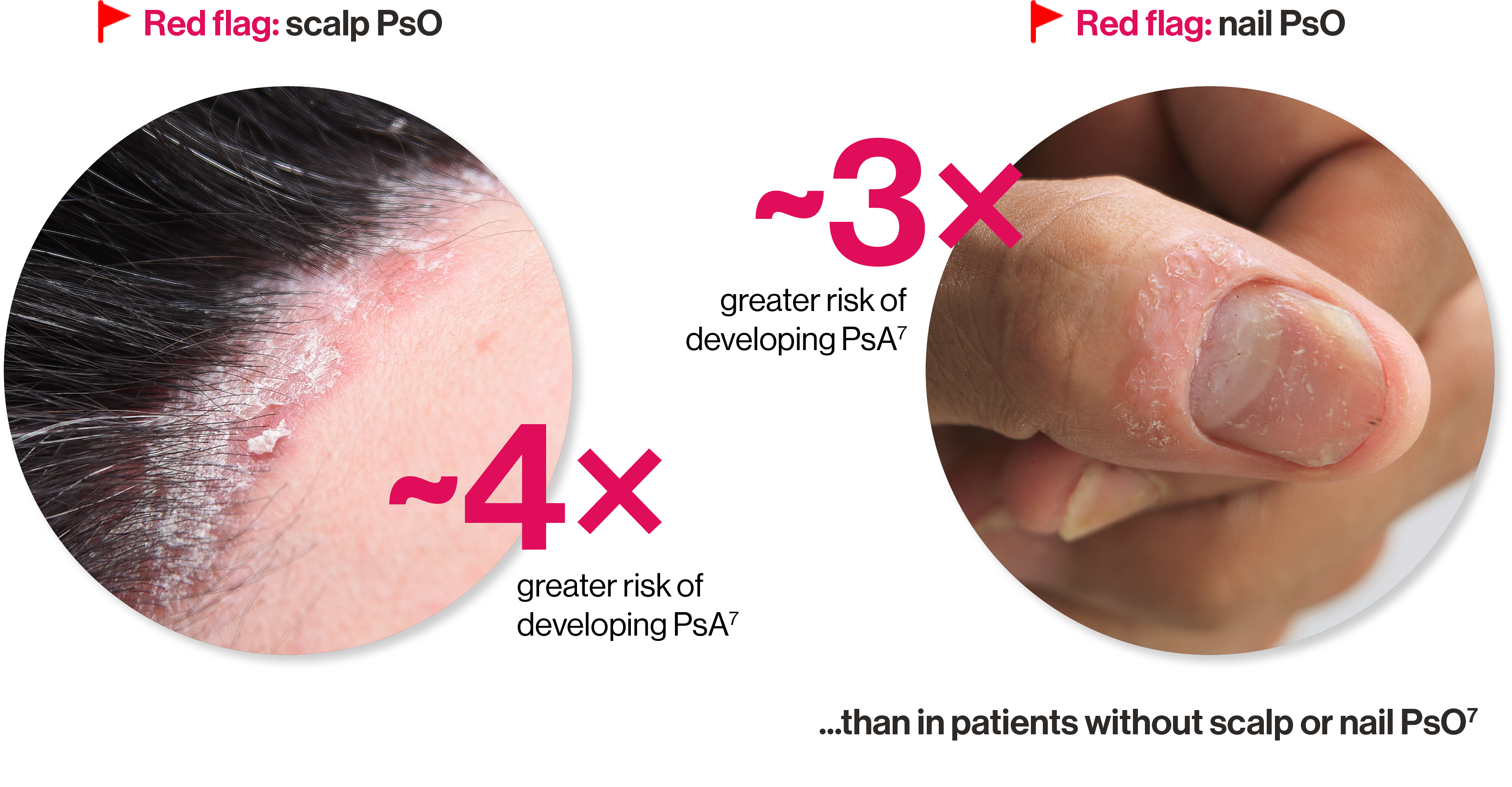
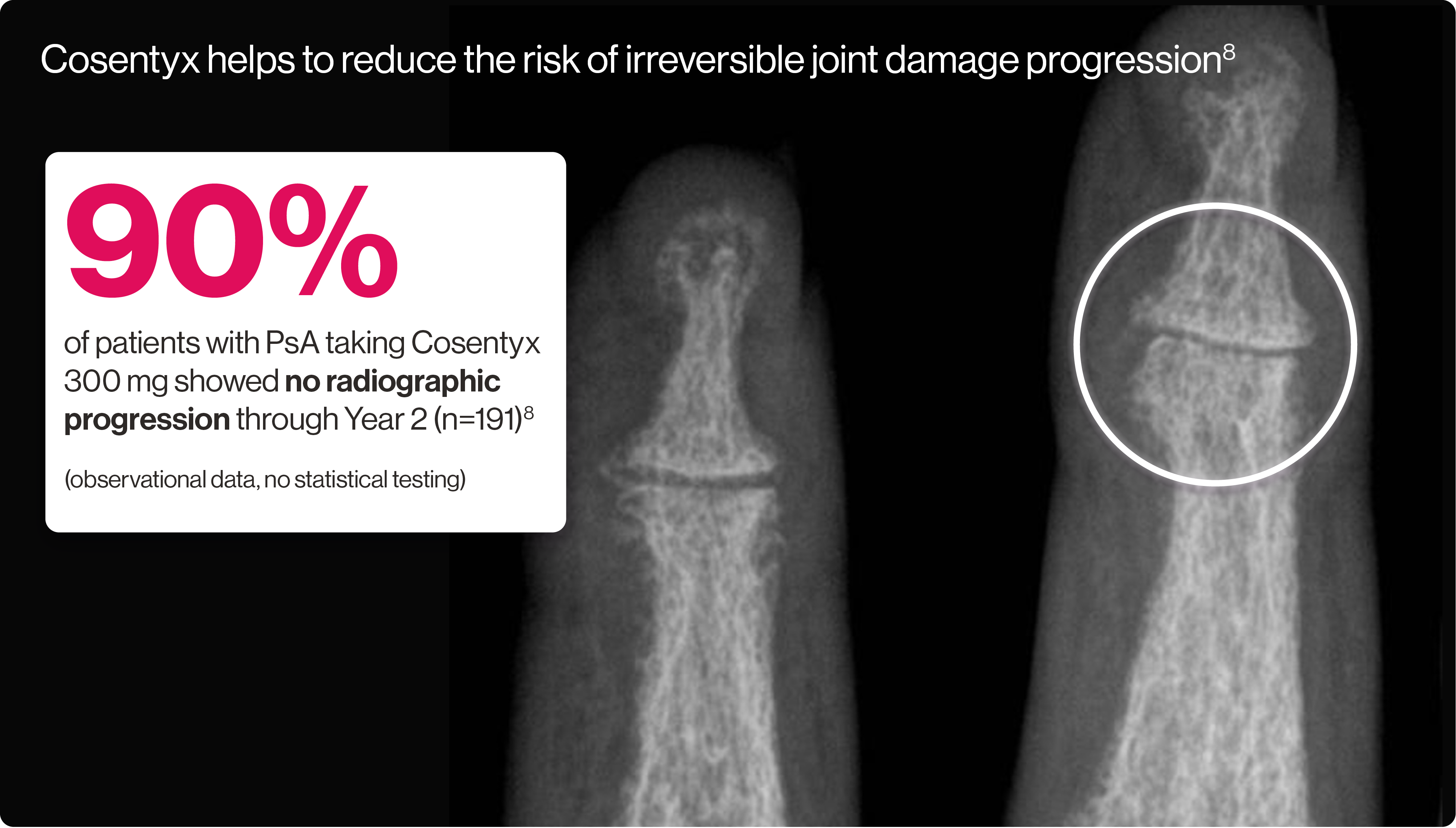
Artist’s representation of early radiographic damage.
FUTURE 5 was a 2-year, randomised, double-blind, placebo-controlled, parallel-group, Phase 3 study in patients with active PsA. The primary endpoint of proportion of patients achieving ACR20 response at Week 16 was met (Cosentyx 300 mg 62.6% vs placebo 27.4%, p<0.0001). The study included 996 patients with active PsA, 222 of whom were taking Cosentyx 300 mg. No structural progression responders are those subjects who have a change in van der Heijde modified total Sharp score relative to baseline of ≤0.5 during the analysis period.9
Cosentyx has clinically proven efficacy in each of the 6 key manifestations of PsA and scalp PsO*1,10–12
Observed and NRI endpoints; not statistically tested.
Nail PsO

73%
sustained reduction in mNAPSI at 2.5 years (observed; n=66)†13,14
TRANSFIGURE: primary endpoint of percentage change from baseline in mean NAPSI score at Week 16 for Cosentyx 300 mg or 150 mg vs placebo was met (−45% for 300 mg, −38% for 150 mg vs −11% for placebo, p<0.001)
Peripheral arthritis

64%
achieved ACR50 at Week 24
(NRI; n=81)II15,16
ULTIMATE: primary endpoint of GLOESS score for Cosentyx vs placebo at Week 12 was met (−9 vs −6 respectively, p=0.004)
Enthesitis

76%
sustained complete resolution at Year 5 (observed; n=51)**17,18
FUTURE 2: primary endpoint of ACR20 response at Week 24 for Cosentyx 300 mg or 150 mg vs placebo was met (54% in 300 mg; 51% in 150 mg; 15% in placebo; p<0.0001)
PsO

55%
sustained PASI 100 at Year 1
(observed; n=41)‡19
MATURE: co-primary endpoints of PASI 75 and IGA 0/1 mod 2011 at Week 12 for Cosentyx Al 300 mg vs placebo were met (95% vs 10% and 76% vs 8% respectively, p<0.0001)
Axial disease

69%
sustained ASAS40 at Year 1 (observed; n=139)¶10
MAXIMISE: primary endpoint of ASAS20 response at Week 12 for Cosentyx 300 mg vs placebo was met (63% vs 31% respectively, p<0.0001)
Dactylitis

88%
sustained complete resolution at Year 5 (observed; n=40)**17,18
FUTURE 2: primary endpoint of ACR20 response at Week 24 for Cosentyx 300 mg or 150 mg vs placebo was met (54% in 300 mg; 51% in 150 mg; 15% in placebo; p<0.0001)
Scalp PsO
53%
sustained PSSI 90 clearance at Week 12
(NRI; n=51)§12
SCALP: primary endpoint of PSSI 90 response at Week 12 for Cosentyx 300 mg vs placebo was met (53% vs 2.0% respectively, p<0.001)
External bodies recommend IL-17 inhibitors as a treatment option for managing PsA in PsO: BAD, BSR and GRAPPA20–22
GRAPPA and BSR
Rheumatological clinical societies and associations recommend IL-17 inhibitors:
✔ GRAPPA 2021 guidance strongly recommends an IL-17 inhibitor as a first-line biologic following inadequate response to DMARDs, across all 6 key manifestations of PsA20
✔ IL-17 inhibitors are recommended as a first-line biologic option by BSR for key manifestations of PsA21
Please note Cosentyx is recommended for patients with PsA when the response to previous disease-modifying anti-rheumatic drug (DMARD) therapy has been inadequate. Please see the SmPC for full information.1
BAD
✔ BAD recommends an IL-17 inhibitor as a first-line biologic for adult patients with PsO and PsA22
Cosentyx is only licensed in adults with PsA (alone or in combination with methotrexate) if there is an inadequate response to a previous DMARD1
In the MATURE clinical trial, results showed FAST and LASTING skin clearance with Cosentyx (FAST = efficacy at 12 weeks and LASTING = efficacy at 52 weeks).19
PASI 100 skin clearance rates over time in biologic-naïve and -experienced patients19
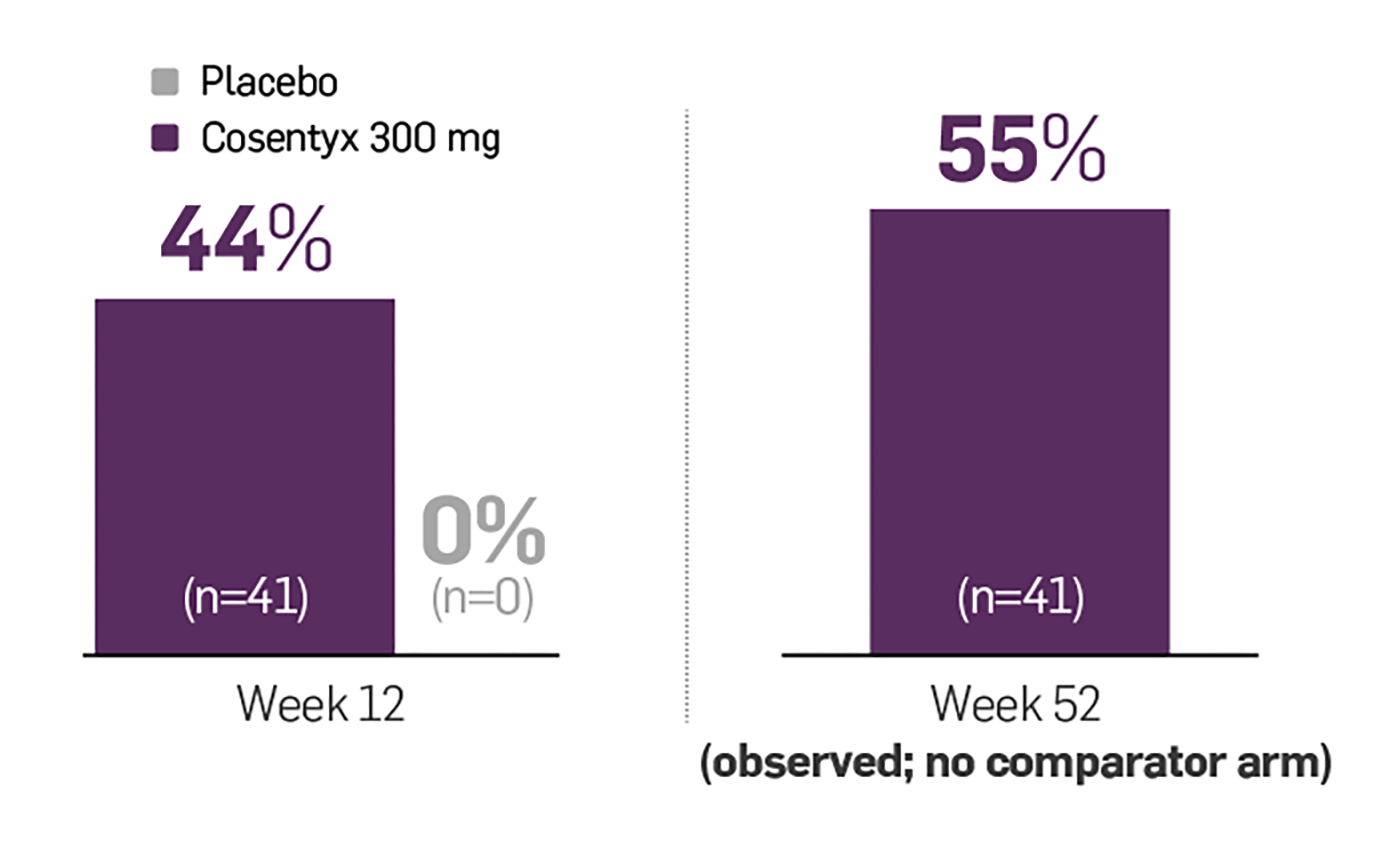
p<0.0001 vs placebo at Week 12.
Adapted from Sigurgeirsson et al. 2022.19
PASI 90 and 100 were key secondary endpoints. Co-primary endpoints: PASI 75 and IGA 0/1 response rates at Week 12 were met (p<0.0001).19

~6 out of 10 patients taking Cosentyx 300 mg achieved PASI 100 at 1 year19
MATURE was a 52-week, multicentre, randomised, double-blind, placebo-controlled, Phase 3 trial (n=122). PASI 75 response rates with Cosentyx via the UnoReady® 300 mg pen were superior to placebo at Week 12 (co-primary endpoint; 95.1% vs 10%, respectively, p<0.0001). IGA 0/1 response rates with Cosentyx via the UnoReady® 300 mg pen were also superior to placebo at Week 12 (co-primary endpoint; 75.6% vs 7.6%, respectively, p<0.0001).19
The SCULPTURE extension study showed long-term persistence for up to 5 years. The SCULPTURE core study did not reach primary end point of non-inferiority of re-treatment as needed vs fixed interval dosing with Cosentyx.23,24
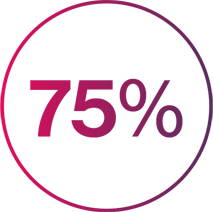
Out of 168 patients receiving Cosentyx in the SCULPTURE study at Year 1, 75% (n=126) of plaque PsO patients remained on Cosentyx at Year 5 in the SCULPTURE extension study23
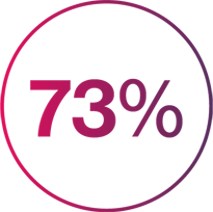
Absolute PASI results showed that at Year 1, 73% of patients reported no impact of PsO on their life, with 66% still reporting this at Year 523
Sustained results23
At Year 1 and through to Year 5, Cosentyx provided an average of 90% change in the a PASI response rate vs baseline (observed).23 Statistical significance not tested.
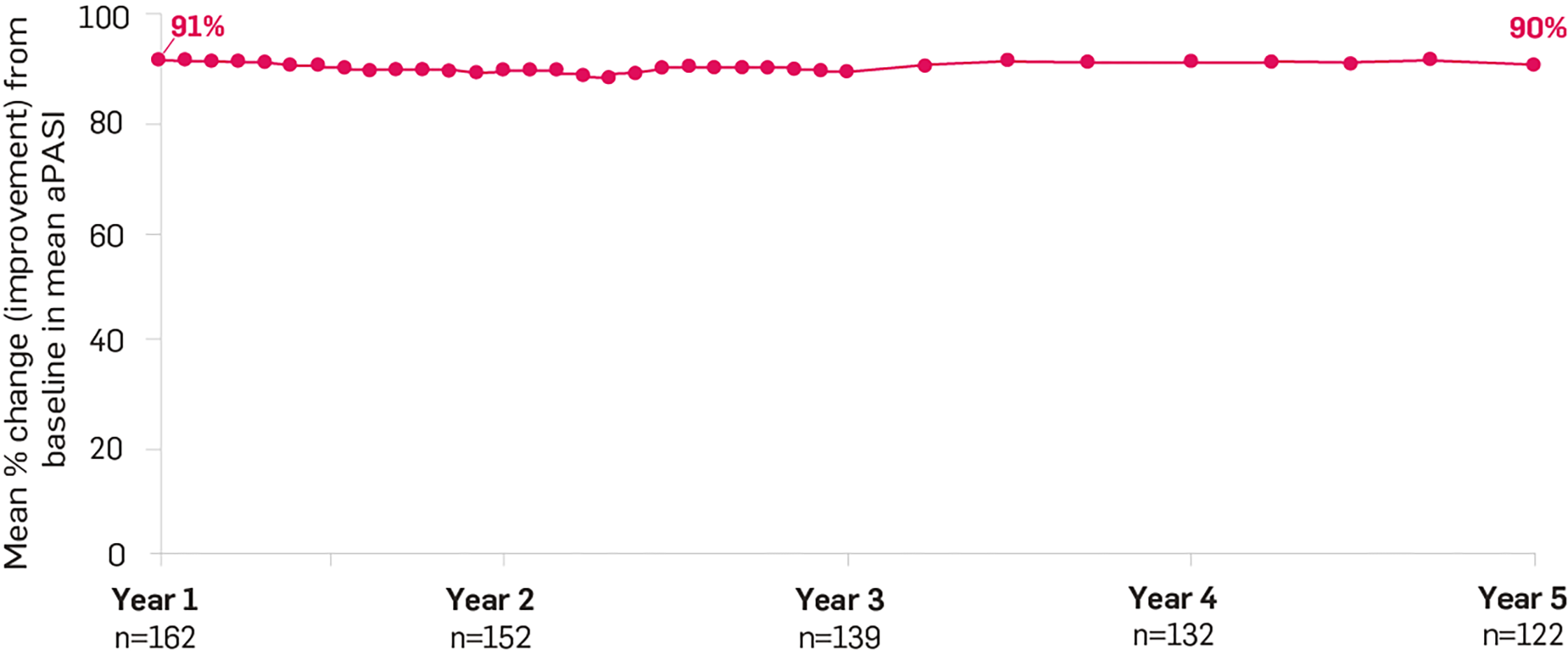
Adapted from Bissonette et al. 2018.23
SCULPTURE extension was a multicentre trial, double-blind from Year 1 through 3, and then open-label through Year 5. The primary endpoint of the core Phase 3 trial was non-inferiority of retreatment as needed vs fixed interval (every 4 weeks) for maintaining PASI 75 to Week 52, which was not established. aPASI ≤5/≤3/≤2/≤1 responses sustained from Year 1 (87.7%, 74.1%, 67.9% and 58.6%, respectively) through to Year 5 (84.4%, 75.4%, 66.4% and 53.3%, respectively).23,24
Please note, 're-treatment as needed' is not a licensed posology. The recommended dose is 300mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe PsO in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active PsA in adult patients (alone or in combination with MTX) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active AS in adults who have responded inadequately to conventional therapy; active nr-axSpA with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe HS (acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active ERA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active JPsA in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*The 6 key manifestations of PsA are joints, axial, skin, enthesitis, dactylitis, and nails.1,10,11
†TRANSFIGURE: the primary endpoint of NAPSI improvement score vs placebo at Week 16 was met (−45.3% for Cosentyx 300 mg vs −10.8% for placebo, p<0.001). Observed data in patients with moderate to severe nail PsO in the 300 mg treatment group (n=66); in the respective 150 mg treatment group (n=67), there was a mean NAPSI improvement of −63.3% sustained at Year 2.13,14
‡MATURE: MATURE was a 52-week, multicentre, randomised, double-blind, placebo-controlled Phase 3 trial (n=122). Co-primary endpoints were PASI 75 and IGA 0/1 response rates at Week 12 vs placebo. PASI 75 was met (95.1% for Cosentyx 300 mg vs 10% for placebo, p<0.0001). IGA 0/1 was also met (75.6% for Cosentyx 300 mg vs 7.6% for placebo, p<0.0001).19
§SCALP (n=102) was a 24-week randomised, double-blind, placebo-controlled, parallel-group, multicentre, Phase 3b study. Patients with moderate to severe scalp PsO received Cosentyx 300 mg or placebo at baseline, Weeks 1, 2, and 3, and then every 4 weeks from Weeks 4 to 20. The final efficacy and safety evaluations were performed at Week 24. The primary endpoint of PSSI 90 vs placebo at Week 12 was met (52.9% for Cosentyx 300 mg vs 2% for placebo, p<0.001).12
IIULTIMATE: nonresponder imputation data in biologic-naïve patients originally randomly assigned to Cosentyx. The primary endpoint of GLOESS score vs placebo at Week 12 was met (−9% for Cosentyx 300 mg vs −6% for placebo, p=0.004).16
¶MAXIMISE: observed data in biologic-naïve patients in the 300 mg treatment group (n=139); in the respective 150 mg treatment group, 65% achieved ASAS40 at Year 1 (n=141). The primary endpoint of ASAS20 vs placebo at Week 12 was met (63% for Cosentyx 300 mg vs 31% for placebo, p<0.0001).10
#FUTURE 2: observed data for the 300 mg treatment group of biologic-naïve patients with this symptom at baseline; 82% in the respective 150 mg group maintained complete resolution of dactylitis through Year 5 (n=28); 75% in the respective 150 mg group maintained complete resolution of enthesitis through Year 5 (n=64). The primary endpoint of ACR20 vs placebo at Week 24 was met (54% for Cosentyx 300 mg vs 15.3% with placebo, p<0.0001).17,18
ACR, American College of Rheumatology; AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis International Society; BAD, British Association of Dermatologists; BSR, British Society for Rheumatology; DMARD, disease-modifying anti-rheumatic drug; ERA, enthesitis-related arthritis; GLOESS, Global OMERACT/EULAR ultrasound synovitis score; GRAPPA, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis; HS, hidradenitis suppurativa; IGA, investigator’s global assessment; IL, interleukin; JPsA, juvenile psoriatic arthritis; mNAPSI, mean nail psoriasis severity index; MTX, methotrexate; NAPSI, nail psoriasis severity index; nr-axSpA, non-radiographic axial spondyloarthritis; NRI, non-responder imputation; PASI, psoriasis area and severity index; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSSI, psoriasis scalp severity index; QoL, quality of life.
References
Cosentyx (secukinumab) Summary of Product Characteristics.
Singh JA, et al. Arthritis Rheumatol 2019;71(1):5–32.
Haroon M, et al. Ann Rheum Dis 2015;74(6):1045–1050.
Menter A, et al. Am J Manag Care 2016;22(Suppl 8):S216–S224.
Gladman DD, et al. Ann Rheum Dis 2005;64(Suppl II):ii14–ii17.
Cigolini C, et al. Clin Exp Rheumatol 2022;40(9):1611–1619.
Wilson FC, et al. Arthritis Rheum 2009;61(2):233–239.
Mease PJ, et al. RMD Open 2021;7(2):e001600.
Mease P, et al. Ann Rheum Dis 2018;77(6):890–897.
Baraliakos X, et al. Ann Rheum Dis 2021;80(5):582–590.
Nash P, et al. Clin Exp Rheumatol 2022;40(5):952–959.
Bagel J, et al. J Am Acad Dermatol 2017;77(4):667–674.
Reich K, et al. Br J Dermatol 2019;181(5):954–966.
Reich K, et al. Br J Dermatol 2021;184(3):425–436.
Boers M, et al. EULAR European Congress of Rheumatology. 2–5 June 2021; Virtual Congress. Poster POS0917.
D’Agostino MA, et al. Rheumatology 2022;61(5):1867–1876.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235.
McInnes IB, et al. Lancet 2015;386(9999):1137–146.
Sigurgeirsson B, et al. Dermatol Ther 2022;35(3):e15285.
Coates LC, et al. Nat Rev Rheumatol 2022;18(8):465–479.
Tucker L, et al. Rheumatology 2022(9);61:e255–e266.
Smith CH, et al. Br J Dermatol 2020(4);183:628–637.
Bissonnette R, et al. J Eur Acad Dermatol Venereol 2018;32(9):1507–1514.
Mrowietz U, et al. J Am Acad Dermatol 2015;73(1):27–36.
UK | June 2025 | FA-11386224
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.
