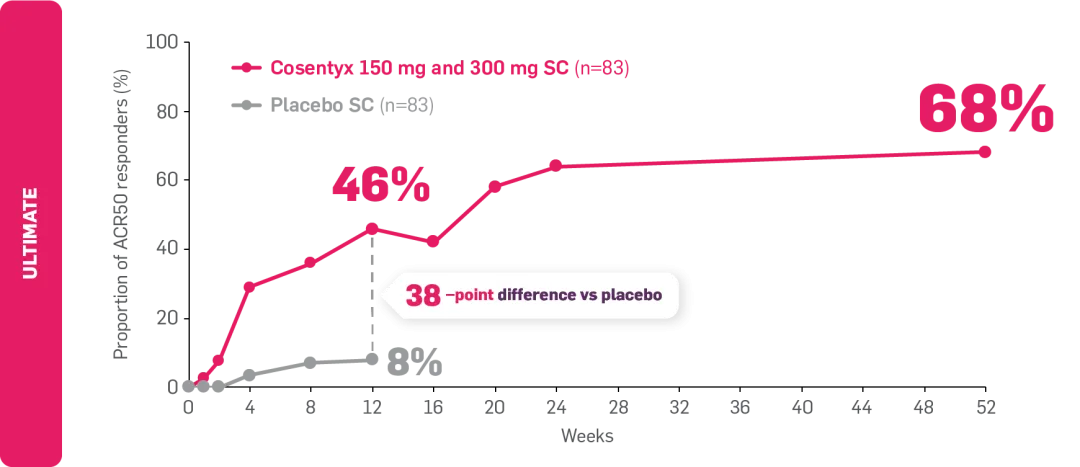
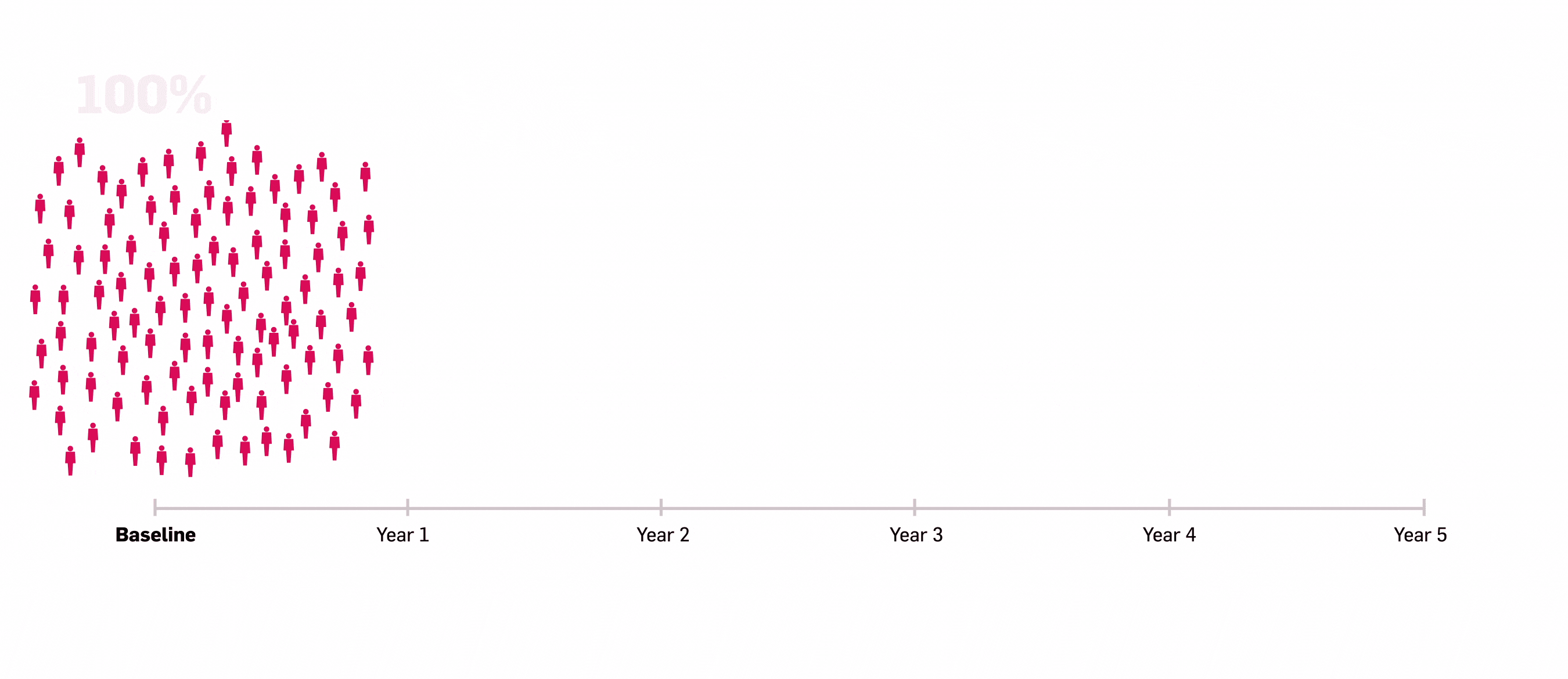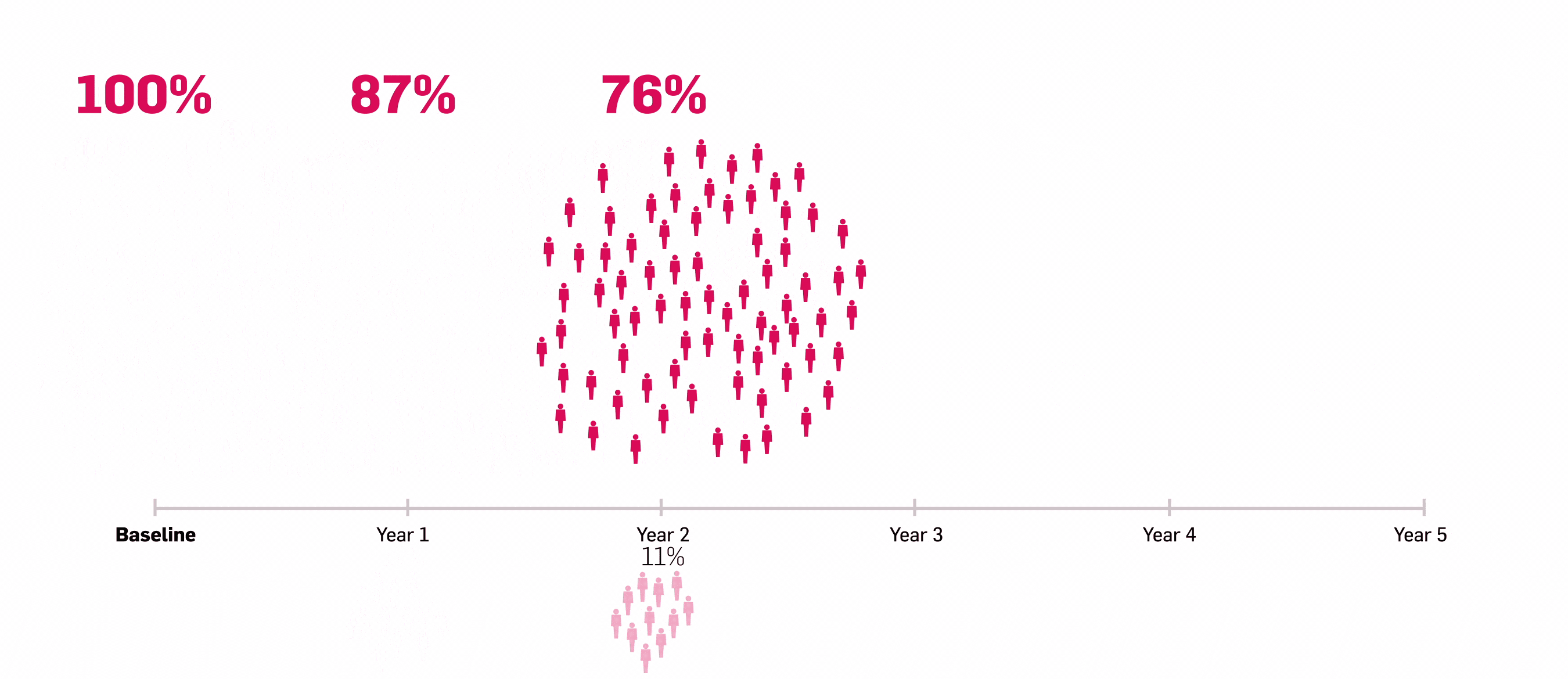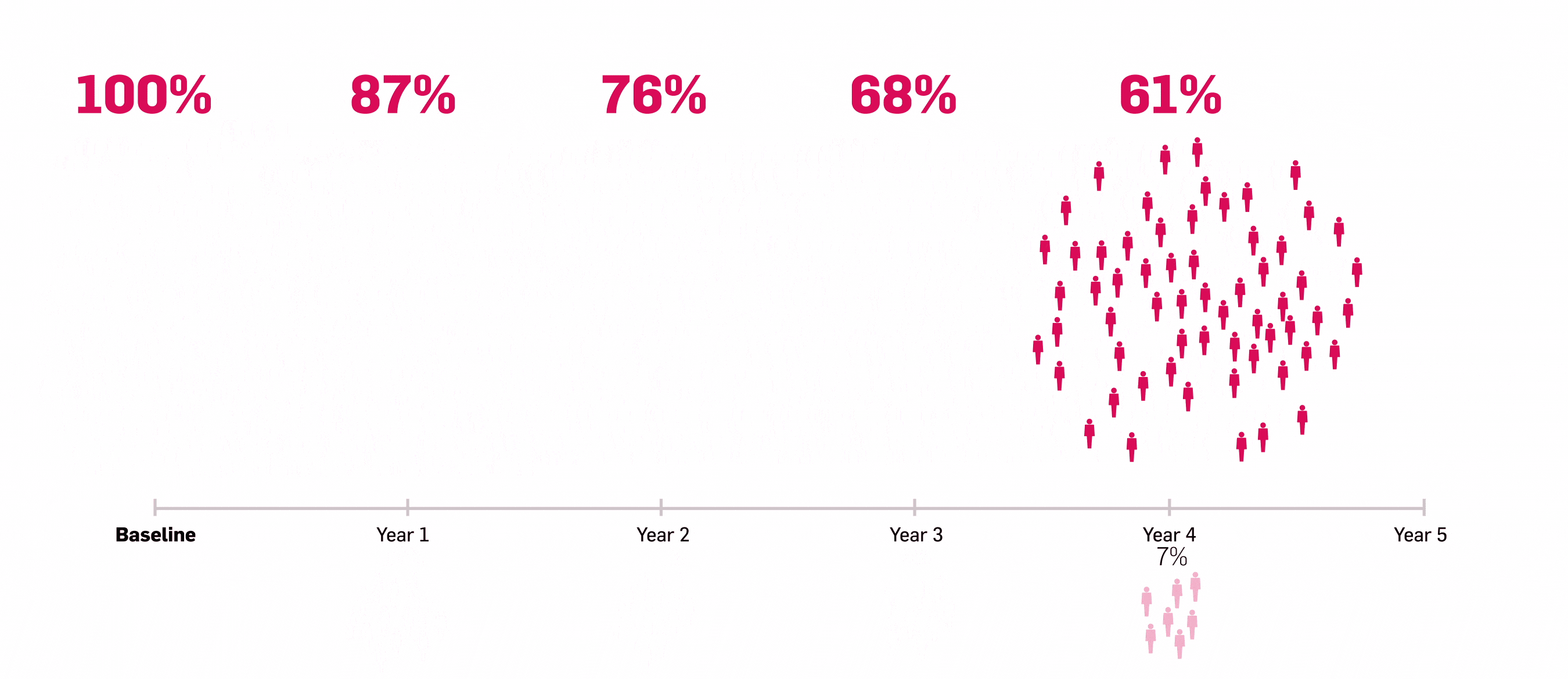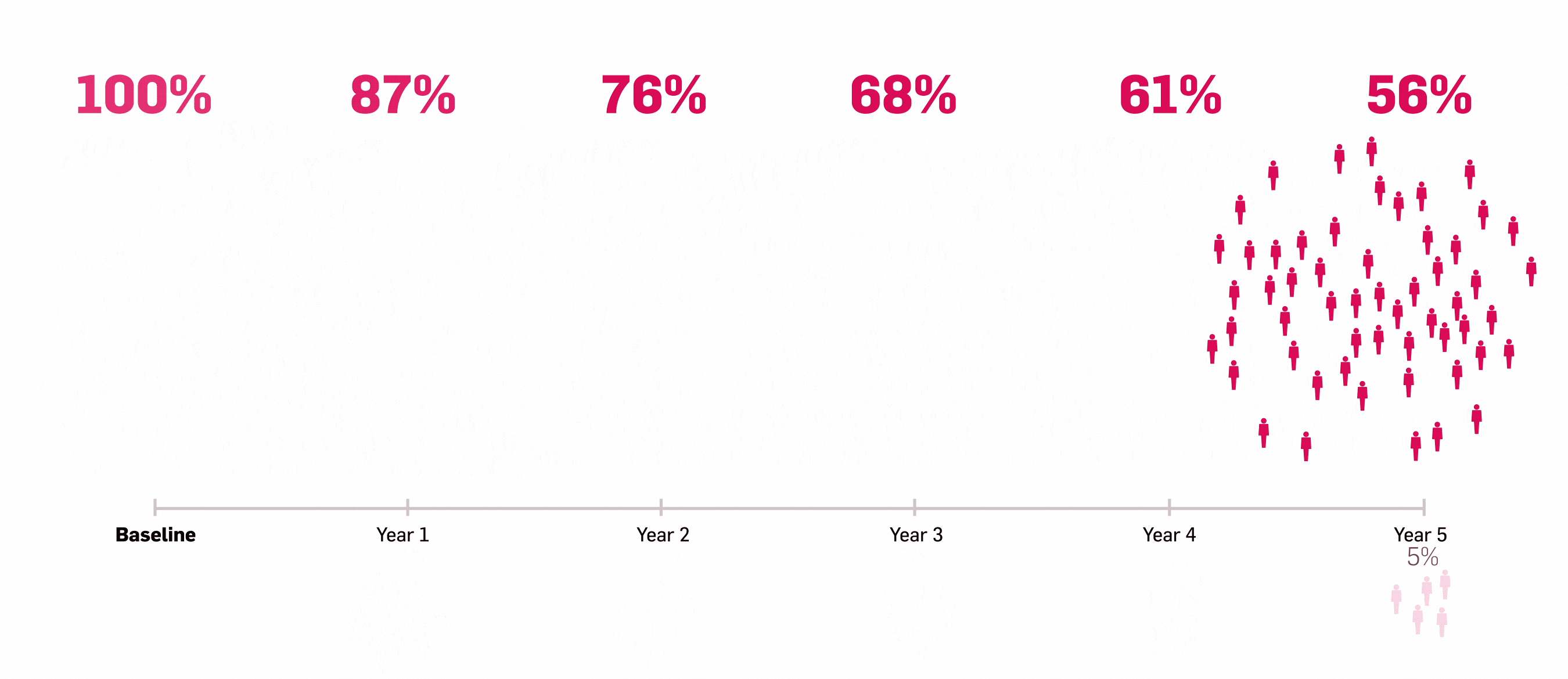*ULTIMATE: Observed data in biologic-naïve patients with PsA receiving Cosentyx 300 mg or 150 mg who were originally randomly assigned to Cosentyx (n=83).2
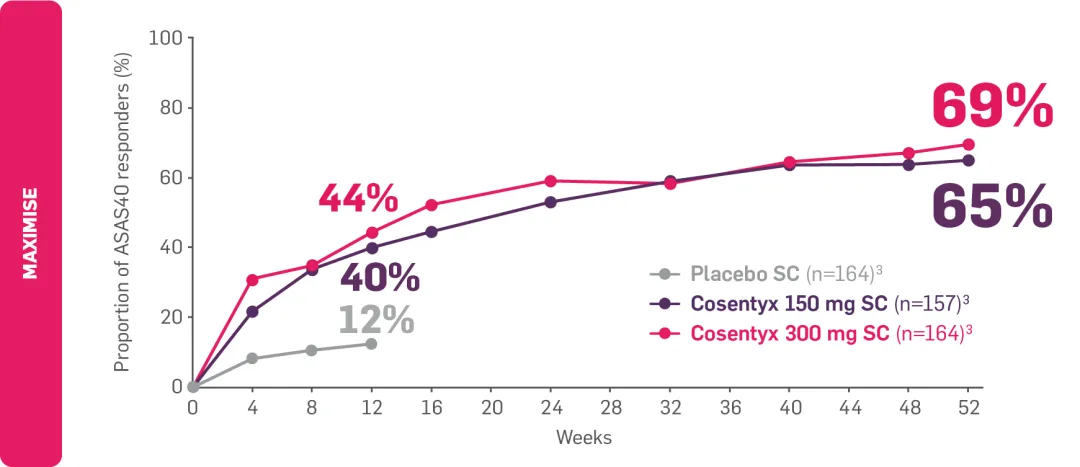
†MAXIMISE: Observed data in biologic-naïve patients with PsA in the Cosentyx 300 mg treatment group (n=139); in the respective Cosentyx 150 mg treatment group, 65% achieved ASAS40 at Year 1 (n=141).3
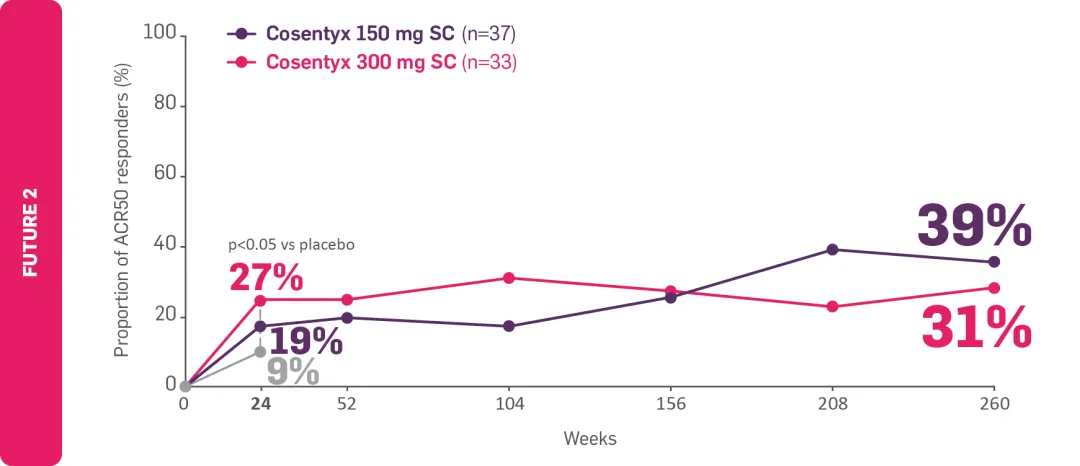
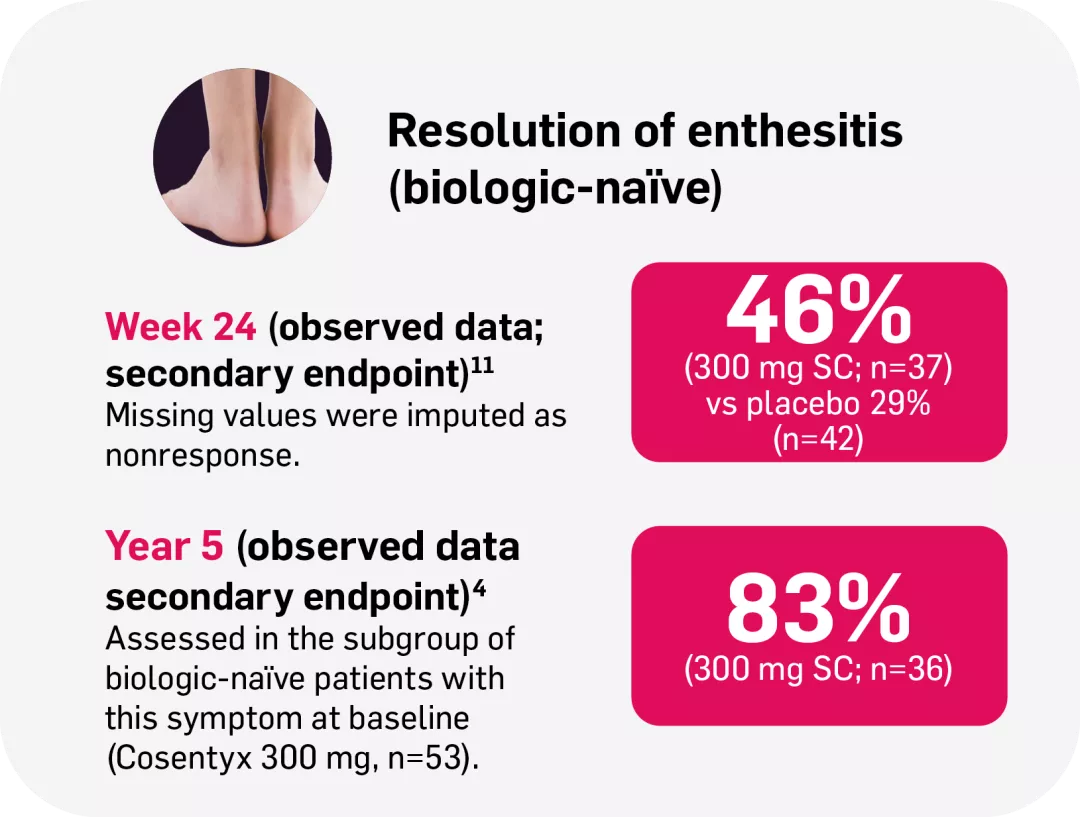
‡FUTURE 2: Observed data for the Cosentyx 300 mg treatment group of biologic-naïve patients with this symptom at baseline, including those originally randomly assigned to Cosentyx and placebo-switchers (n=36); 76% in the respective Cosentyx 150 mg group maintained complete resolution of enthesitis through Year 5 (n=45).4
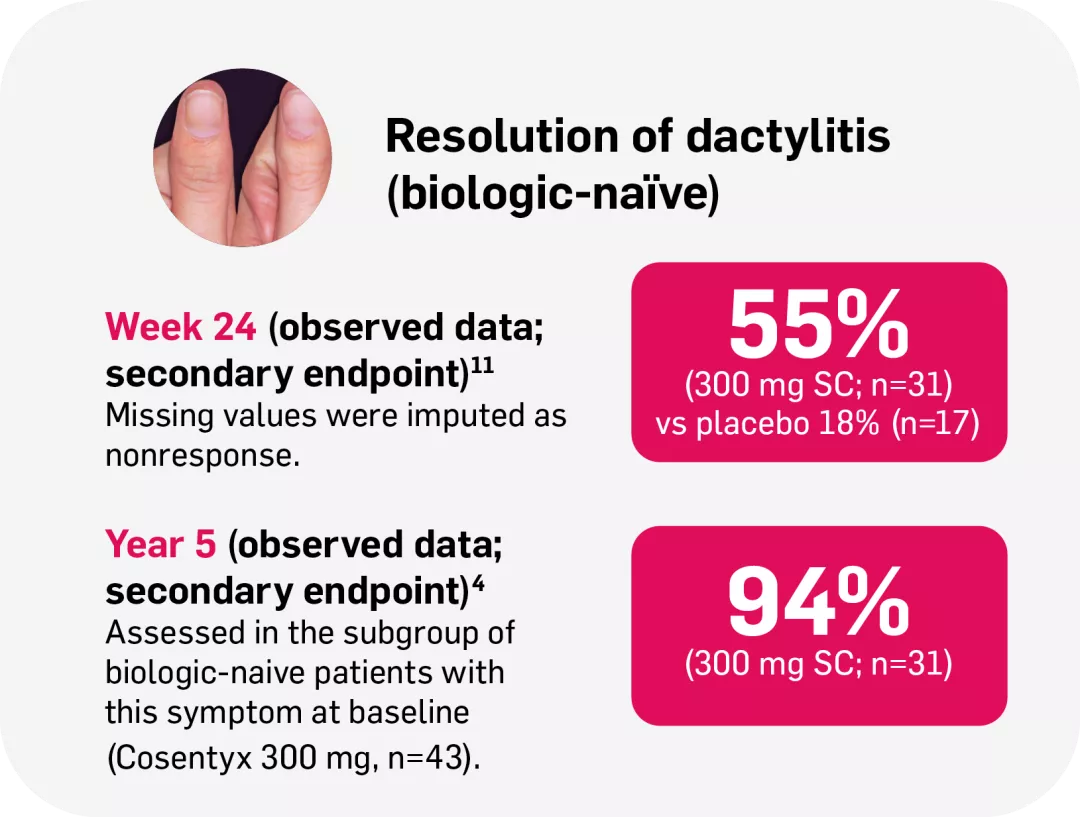
§FUTURE 2: Observed data for the Cosentyx 300 mg treatment group of biologic-naïve patients with this symptom at baseline, including those originally randomly assigned to Cosentyx and placebo-switchers (n=31); 86% in the respective Cosentyx 150 mg group maintained complete resolution of dactylitis through Year 5 (n=22).4
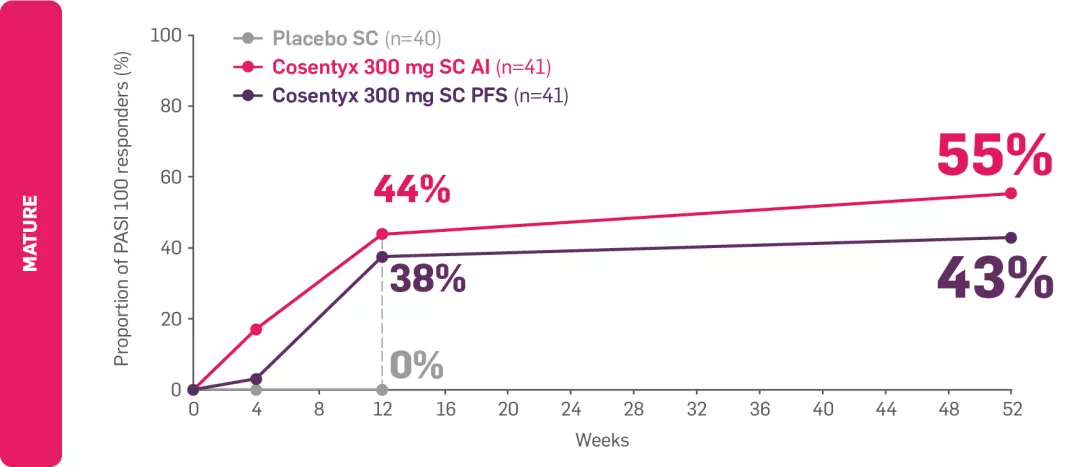
¶MATURE: Percentage of patients with moderate-to-severe PsO at baseline by visit (multiple imputation) for patients receiving Cosentyx 300 mg by autoinjector (n=41); in the Cosentyx 300 mg prefilled syringe group (n=41), 43% achieved PASI 100 at Year 1.6
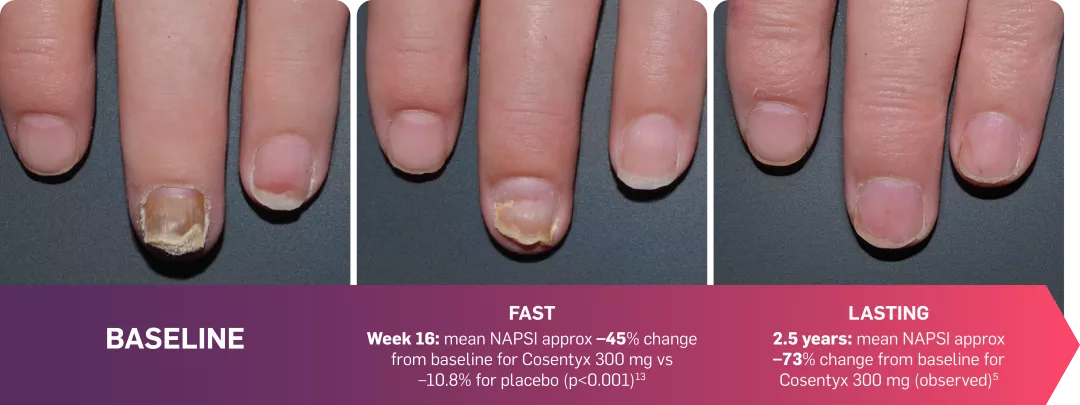
IITRANSFIGURE: Observed data in patients with moderate to severe nail PsO in the Cosentyx 300 mg treatment group (n=66; NRI; observed data).5
ACR, American College of Rheumatology; AI, autoinjector; AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis international Society; axSpA, axial spondyloarthritis; BSA, body surface area; ERA, enthesitis-related arthritis; EULAR, European Alliance of Associations for Rheumatology; GLOESS, Global OMERACT-EULAR synovitis score; HS, hidradenitis suppurativa; IGA, investigator’s global assessment; IR, inadequate responder; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; mNAPSI, modified nail psoriasis severity index; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; NRI, non-responder imputation; OMERACT, outcome measures in rheumatology; PASI, psoriasis area and severity index; PFS, pre-filled syringe; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSSI, psoriasis scalp severity index; SC, subcutaneous; SmPC, summary of product characteristics; TNFi-IR, tumour necrosis factor inhibitor-inadequate responder.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
D’Agostino MA, et al. Semin Arthritis Rheum 2023;63:152259.
Baraliakos X, et al. Ann Rheum Dis 2021;80(5):582–590.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235 and supplementary appendix.
Reich K, et al. Br J Dermatol 2021;184(3):425–436.
Sigurgeirsson B, et al. Dermatol Ther 2022;35(3):e15285.
Boers M, et al. Poster POS0197B. EULAR European Congress of Rheumatology. 2–5 June 2021; virtual.
D’Agostino MA, et al. Rheumatology (Oxford). 2022;61(5):1867–1876 and supplementary appendix.
McInnes IB, et al. Rheumatology (Oxford). 2017;56(11):1993–2003.
Novartis Data on File. CAIN457F3302 (MAXIMISE) Clinical Study Report. August 2020.
Kavanaugh A, et al. J Rheumatol 2016;43(9):1713–1717.
McInnes IB, et al. Lancet 2015;386(9999):1137–1146.
Reich K, et al. Br J Dermatol 2019;181(5):954–966.
Bagel J, et al. J Am Acad Dermatol 2017;77:667–674.
Kiltz U et al. Abstract 1840456. American College of Rheumatology (ACR) Convergence, 14–19 November 2024; Washington, DC, US.
Kiltz U, et al. Adv Ther 2020;37(6):2865–2883.
Kiltz U, et al. Rheumatol Ther 2022;9(4):1129–1142.
UK | July 2025 | FA-11426985-1