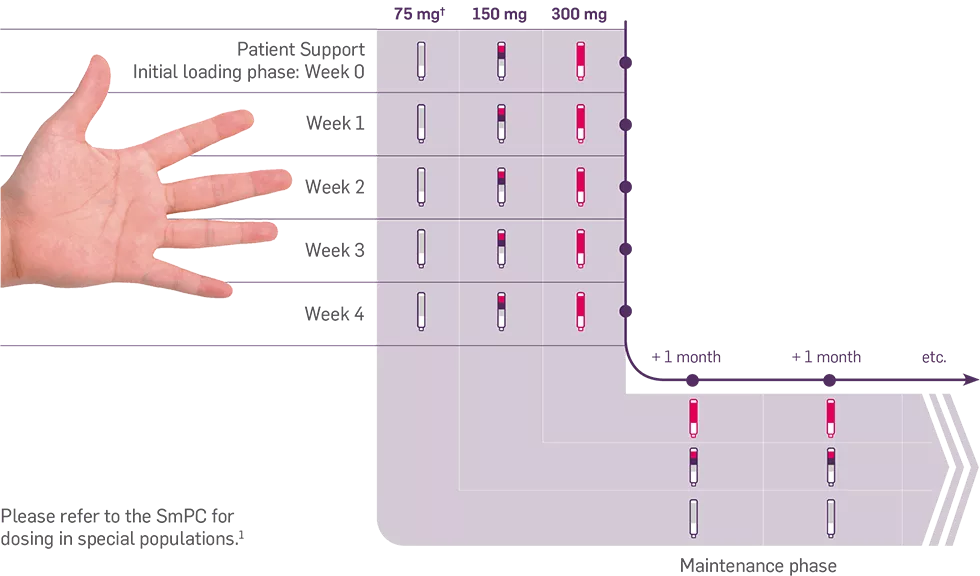
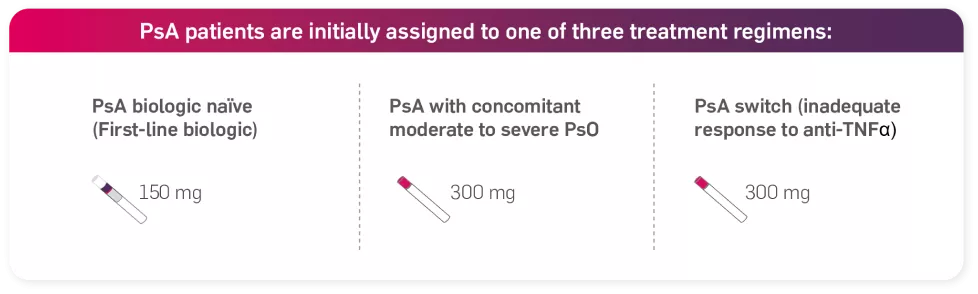
For patients with concomitant moderate to severe plaque psoriasis, the recommended dose is 300 mg of Cosentyx by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, a maintenance dose of 300 mg every 2 weeks may provide additional benefit for patients with a body weight of 90 kg or higher.1
For patients who are anti-TNFα inadequate responders (IR), the recommended dose is 300 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing.1
Each 300 mg dose is given as one subcutaneous injection of 300 mg or as two subcutaneous injections of 150 mg.1
For other patients, the recommended dose is 150 mg by subcutaneous injection with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.1
Special populations
Elderly patients (aged 65 years and over): No dose adjustment is required.1
Renal impairment/hepatic impairment: Cosentyx has not been studied in these patient populations. No dose recommendations can be made.1
Paediatric population: The safety and efficacy of Cosentyx in children with plaque psoriasis and in the JIA categories of ERA and JPsA below the age of 6 years have not been established.
The safety and efficacy of Cosentyx in children below the age of 18 years in other indications have not yet been established. No data are available.1