Disease burden in psoriatic arthritis (PsA)
Cosentyx® (secukinumab) is indicated for the treatment of: moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adults (alone or in combination with methotrexate [MTX]) who have responded inadequately to disease-modifying anti-rheumatic drug therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
PsA is a highly unpredictable disease
Patients present with 1 or more manifestations initially, but may develop more over time, resulting in a challenging balancing act.2,3
Joints

Up to 96% of patients
with PsA may have peripheral arthritis4
Axial
Up to 50% of patients
with PsA may have axial disease4
Enthesitis

Up to 50% of patients
with PsA may have enthesitis4
Dactylitis

Up to 50% of patients
with PsA may have dactylitis4
Nails
Up to 83% of patients
with PsA may have nail disease5
Skin
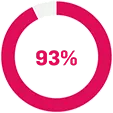
Up to 80% of patients
with PsA may have psoriasis6
These are representative patient images.
Are you considering all manifestations, including skin, in your PsA treatment plans?
Prevalence of PsA manifestations in biologic initiators (% of patients; n=354)2

Adapted from Ogdie A, et al. 2021.2
PsA is associated with an increased risk of cardiovascular diseases (CVD)7
Prevalence of comorbidities in patients with PsA
PsA cohort: n=94,302; continuously enrolled population: n=47,438. Study based on patient data from 2008–2015, extracted from a large US national claims database.8
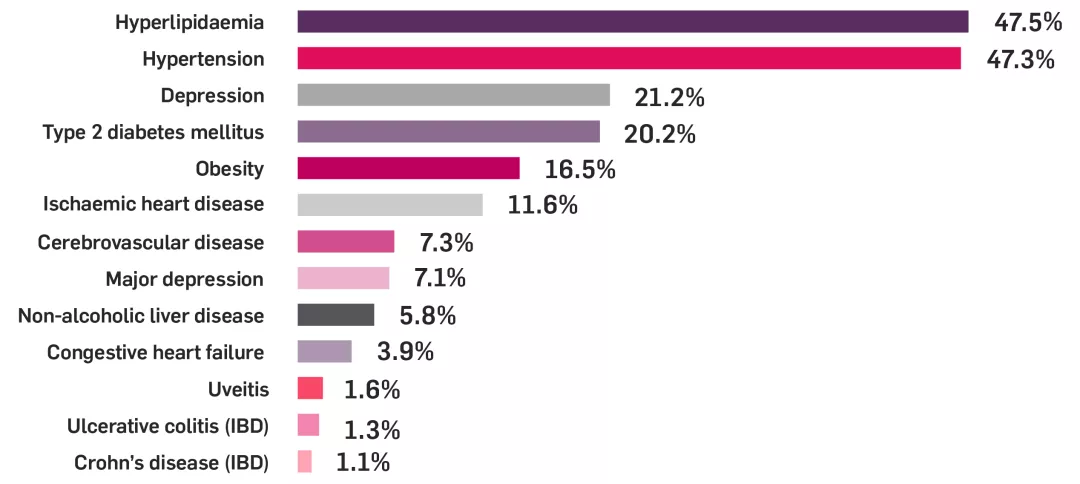
Adapted from Shah K, et al. 2017.8
Cosentyx is not licensed for the treatment of uvetitis. Cosentyx is not recommended in patients with IBD; discontinue treatment if signs and symptoms develop. Please consult the SmPC before prescribing.1
Think skin…
Up to 80% of patients with PsA may have psoriasis.6
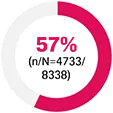
Many of your patients with PsO may still be experiencing an unmet need.9
Almost 80% of patients with PsO will experience scalp psoriasis10
Patients with scalp psoriasis have a 4x higher risk of irreversible joint damage11
Skin involvement may worsen overall disease activity and patient-reported outcomes.12
Patients with PsA involving both skin and joints found that skin severity was linked to:12

The number of PsA symptoms

The level of pain reported by patients

The number of joints affected
When was the last time your patient told you about their skin?
*Results based on a worldwide survey developed and funded by Novartis of 8338 patients with moderate to severe PsO from 31 countries. Patients reported being on a range of current treatments, including topical treatment with prescription, topical treatment without prescription, prescription pill treatment, phototherapy/UV light therapy, prescription injection therapy, other, or none of the above.9
Think nails…
Because of my nail psoriasis, I:14
“Cannot lead a normal working life”
“Avoid touching other people”
“Feel depressed or less self-confident”
These are representative patient quotes aligned to questions asked to calculate a nail assessment in psoriasis and psoriatic arthritis quality of life (NAPPA-QOL) score.14
Patients with nail psoriasis have a 3x higher risk of developing PsA.11
When was the last time your patient told you about their nails?
Think joints…
Joint damage can develop in as little as 6 months from symptom onset.15
Treatment delay can lead to irreversible damage, significantly impacting patient QoL in the future.16
Find out more about the efficacy of Cosentyx across all manifestations, including skin, in eligible patients with PsA
Switching to another TNFi may not provide the same amount of control as the first18
Results are based on an observational Danish registry study (first treatment course N=1422; second treatment course n=548):18
FUTURE 2 study:19
The primary endpoint (ACR20 response at Week 24) was met (Cosentyx 300 mg: 54% vs placebo: 15%; p<0.0001).19
Cosentyx may provide a treatment option for eligible patients with PsA who have had an inadequate response to prior TNFi therapies19
Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
FUTURE 2: was a multicentre, randomised, double-blind, placebo-controlled Phase III trial that evaluated 397 adult patients with active PsA (≥3 swollen and ≥3 tender joints) despite use of NSAIDs, corticosteroids, or DMARDs. Patients received Cosentyx 75 mg (n=99) [Cosentyx 75 mg is not a licensed dose within this population], 150 mg (n=100), 300 mg (n=100), or placebo SC (n=98) at Weeks 0, 1, 2, 3, and 4, followed by the same dose every 4 weeks. Patients who received placebo were re-randomised to receive Cosentyx 150 mg or 300 mg every 4 weeks at Week 16 or Week 24 based on responder status. The primary endpoint was the percentage of patients with ACR20 response at Week 24 — ACR20 results at Week 24: 54% in 300 mg; 15% in placebo; p<0.0001 for placebo vs all doses. At baseline, approximately 65% of patients were biologic-naïve and 47% of patients were treated with concomitant methotrexate.19
AS, ankylosing spondylitis; BSR, British Society for Rheumatology; CVD, cardiovascular disease; ERA, enthesitis-related arthritis; EULAR, European Alliance of Associations for Rheumatology; GRAPPA, Group for Research and Assessment of Psoriasis and Psoriatic Arthritis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; IR, inadequate response; JPsA, juvenile psoriatic arthritis; MTX, methotrexate; NAPPA-QOL, nail assessment in psoriasis and psoriatic arthritis quality of life; NNT, number needed to treat; nr-axSpA, non-radiographic axial spondyloarthritis; PsA, psoriatic arthritis; PsO, plaque psoriasis; QoL, quality of life; SC, subcutaneous; SmPC, summary of product characteristics; TNFi, tumour necrosis factor inhibitor; UV, ultraviolet.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Ogdie A, et al. J Rheumatol 2021;48(5):698–706.
Tiwari V, et al. In: StatPearls. Psoriatic Arthritis. StatPearls Publishing; 2024. Available at: https://www.ncbi.nlm.nih.gov/books/NBK547710/ [Accessed June 2025].
Ritchlin, CT et al. N Engl J Med 2017;376(10):957–970.
Lee S, et al. P T 2010;35(12):680–689.
Gottlieb AB, et al. J Dermatolog Treat 2006;17(5):279–287.
Polachek A, et al. Arthritis Care Res 2017;69(1):67–74.
Shah K, et al. RMD Open 2017;3(2):e000588.
Armstrong A, et al. J EurAcad Dermatol Venereol 2018;32(12):2200–2207.
Wang TS, Tsai TF. Am J Clin Dermatol 2017;18(1):17-43.
Wilson FC et al. Arthritis Rheum 2009;61(2):233–239.
Vlam K, et al. Rheumatol Ther 2018;5(2):423–436.
Radtke MA, et al. J Dtsch Dermatol Ges 2013;11(3):203–220.
Nail Assessment in Psoriasis and Psoriatic Arthritis. Modular condition-specific questionnaire. Available at: https://nappa-online.com/wp-content/uploads/nappa_short_information.pdf [Accessed June 2025].
Haroon M, et al. Ann Rheum Dis 2015;74(6):1045–1050.
Mease PJ, et al. Drugs 2014;74(4):423–441.
James L, et al. Ther Adv Musculoskelet Dis 2024;16:1–23.
Glintborg B et al. Arthritis Rheum 2013;65(5):1213–1223.
McInnes IB, et al. Lancet 2015;386(9999):1137–1146
Tucker L et al. Rheumatology (Oxford) 2022;61(9):e255–e266.
Gossec L et al. Ann Rheum Dis 2024;83(6):706–719.
Coates LC et al. Net Rev Rheumatol 2022;18(8):465–479.
UK | June 2025 | FA-11426969
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.