Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
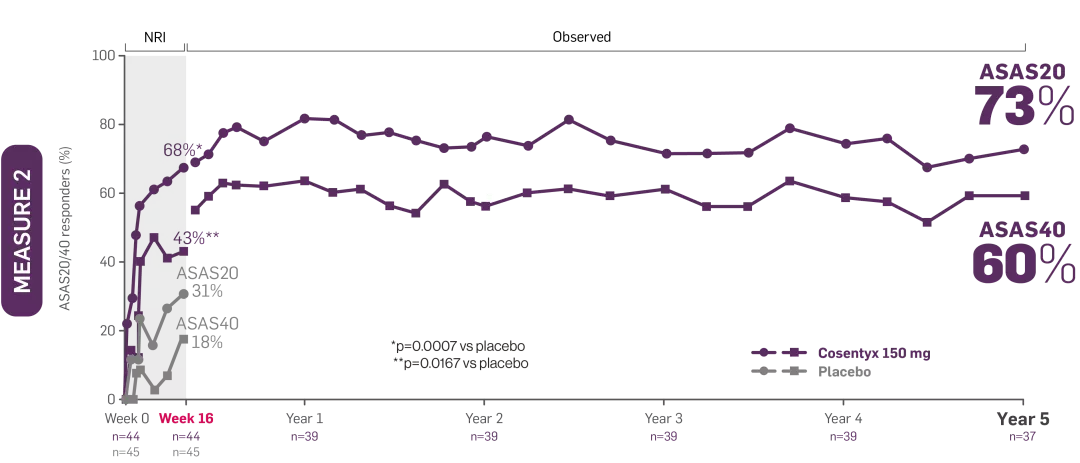
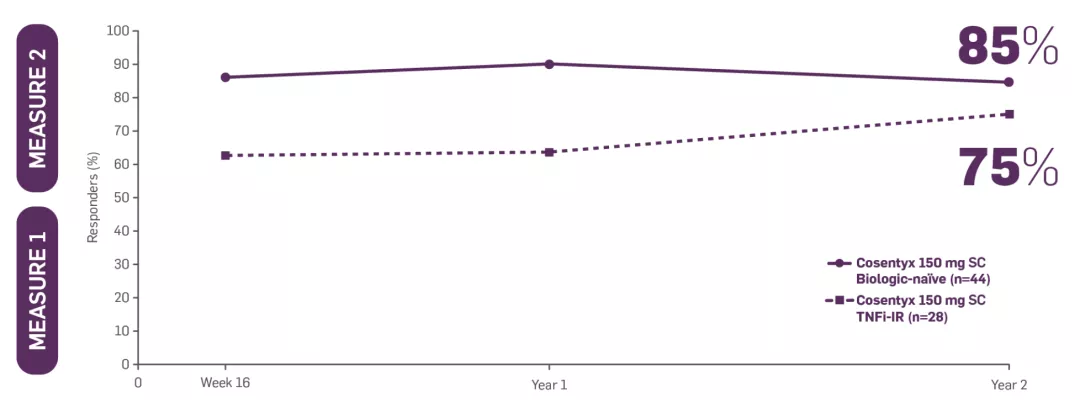
*MEASURE 2 included patients who did not switch treatment and who switched to open-label or standard of care after Week 20.
†Patients on Cosentyx 150 mg received a 150 mg loading dose.
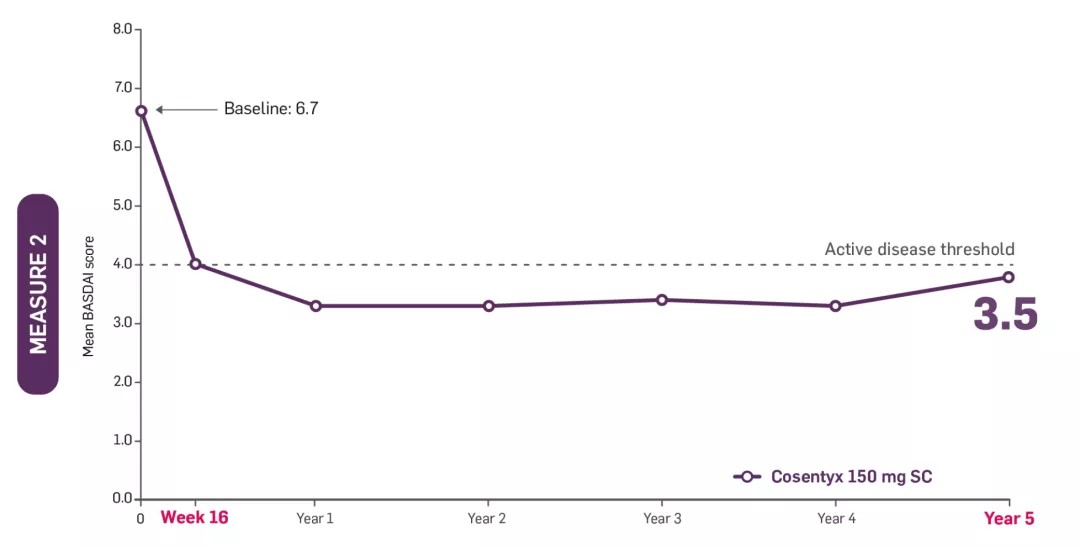
‡MEASURE 2 BASDAI data presented were an exploratory endpoint; no statistical analysis performed. Patients on Cosentyx 150 mg received a 150 mg loading dose.
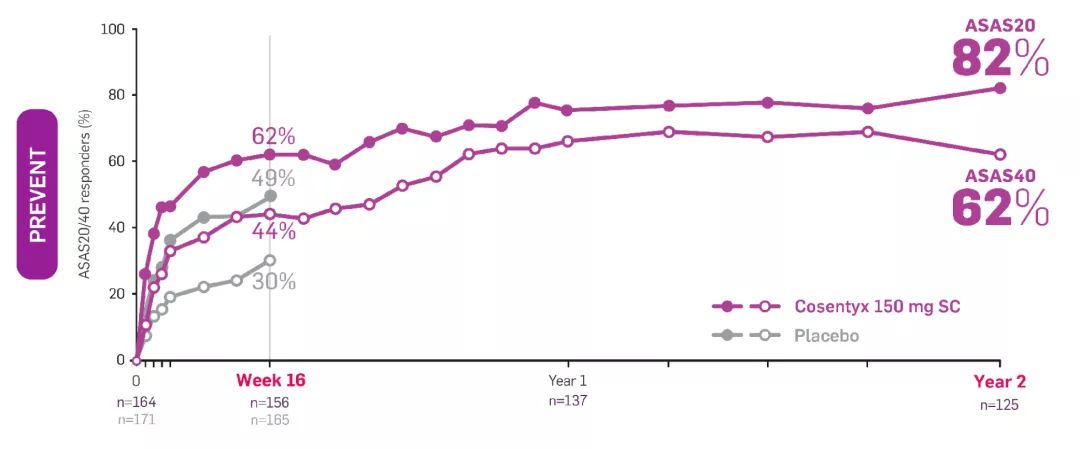
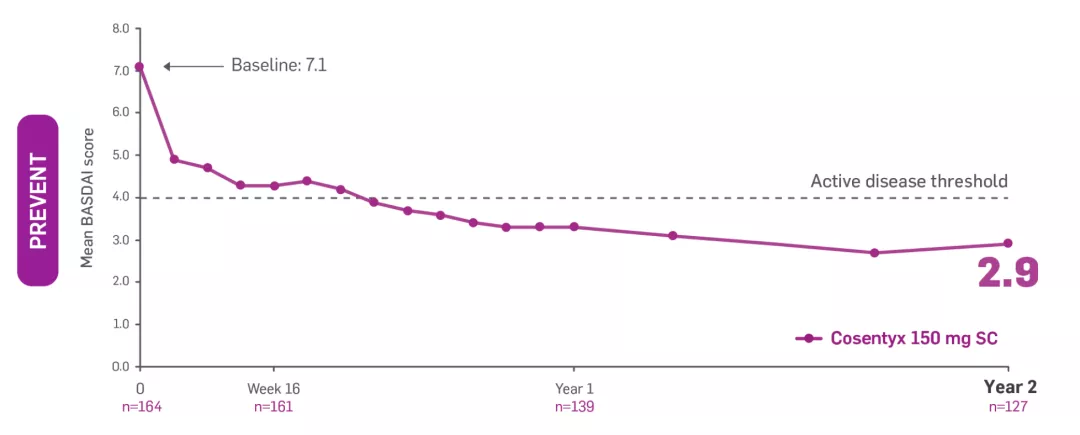
§PREVENT BASDAI data presented were an exploratory endpoint; no statistical analysis performed. Includes patients who did not switch treatment and who switched to open-label or standard of care after Week 20. The proportion of patients who switched to either open-label Cosentyx or standard of care between Weeks 20 and 52, based on clinical judgement of disease activity by the investigator and the patient, was 50.8% (94/185, with 94 switching to open-label and 2 subsequently switching to standard of care treatment with anti-TNF) in 150 mg loading dose.
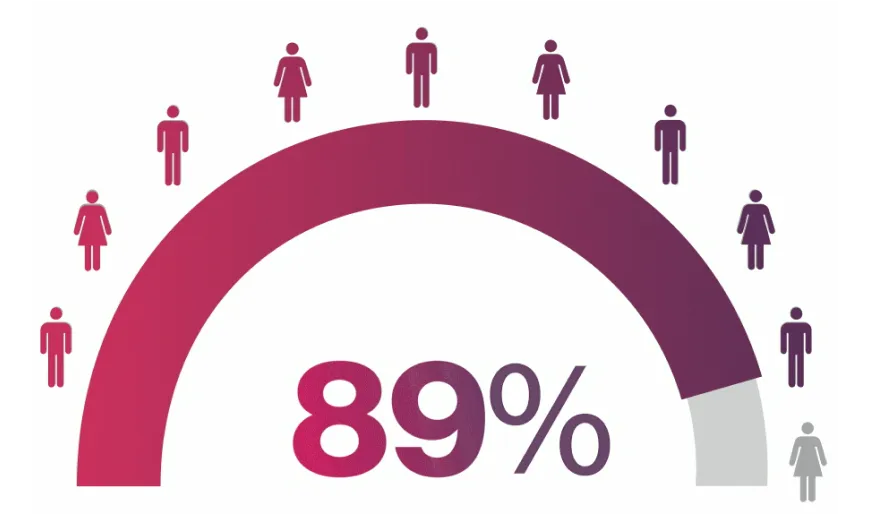
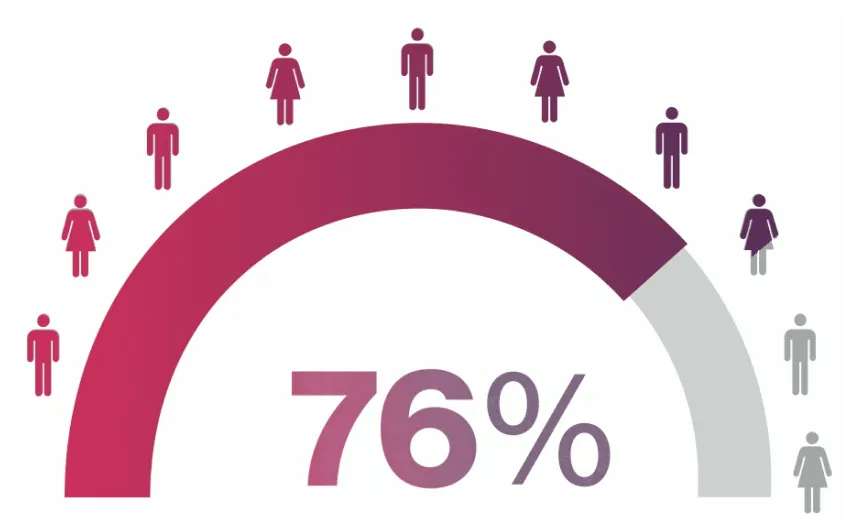
¶MEASURE 1/2 FACIT-F data were an exploratory endpoint. FACIT-F response was defined as improvement (increase in FACIT-F score) by ≥4.0 points from baseline. Data from the MEASURE 1 study are from patients who entered the MEASURE 1 long-term extension study at Week 104.
AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis international Society; axSpA, axial spondyloarthritis; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BHPR, British Health Professionals in Rheumatology; BSR, British Society for Rheumatology; CI, confidence interval; ERA, enthesitis-related arthritis; EULAR, European Alliance of Associations for Rheumatology; FACIT-F, functional assessment of chronic illness therapy-fatigue; HS, hidradenitis suppurativa; IL-17, interleukin 17; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; MMRM, mixed model repeated measures; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; NRI, non-responder imputation; PsA, psoriatic arthritis; PsO, plaque psoriasis; SC, subcutaneous; SmPC, Summary of Product Characteristics; TNF, tumour necrosis factor; TNFi, tumour necrosis factor inhibitor; TNFi-IR, tumour necrosis factor inhibitor-inadequate responder; VAS, visual analogue scale.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Marzo-Ortega H, et al. Lancet Rheumatol 2020 (6);2:e339–e346.
Deodhar A, et al. Arthritis Rheumatol 2021;73(1):110–120.
Novartis Data on File. CAIN457F2310 Data Analysis Report. June 2019.
Novartis Data on File. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. January 2020.
Novartis Data on File. CAIN457F2310 (MEASURE 2): Nocturnal Back Pain. February 2021.
Deodhar A, et al. Clin Exp Rheumatol 2019;37(2):260–269.
Taurog JD, et al. N Engl J Med 2016;375(13):1303.
Marzo-Ortega H, et al. Arthritis Care Res 2017;69(7):1020–1029 and supplementary tables.
Baeten D, et al. N Engl J Med 2015;373(26):2534–2548.
Novartis Data on File. CAIN457H2315 Data Analysis Report. August 2021.
Ramiro S, et al. Ann Rheum Dis 2023;82(1):19–34.
Hamilton L, et al. Rheumatol 2017;56(2):313–316.
Novartis Data on File. Rheumatology UK 22.
Novartis Data on File. Rheumatology UK 23.
Novartis Data on File. Rheumatology UK 268.
Novartis Data on File. Rheumatology UK 269.
Zochling J. Arthritis Care Res (Hoboken) 2011;63(suppl 11):S47–S58.
Novartis Data on File. CAIN457H2315 Data Analysis Report. April 2020.
Kvien TK, et al. Ann Rheum Dis 2017;76(suppl. 2):355–356. Abstract THU0393.
Gaffney K, et al. Rheum Adv Prac 2023;7(2):rkad055.