Prescribing information (external link)


How might you treat patients with comorbidities?
Cosentyx® (secukinumab) is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adults (alone or in combination with methotrexate [MTX]) who have responded inadequately to disease-modifying anti-rheumatic drug therapy.1
Full indications for Cosentyx can be found here
Cosentyx Summary of Product Characteristics (SmPC) can be found here.
Cosentyx is intended for use under the guidance and supervision of a physician experienced in the diagnosis and treatment of conditions for which Cosentyx is indicated.
Meet Mark
Mark is a 55-year-old man with PsA and cardiovascular (CV) comorbidities. Mark presents with the following:

The images on this page do not depict real patients. For illustrative purposes only.
Compared with the general population, patients with PsA have:*2

43% increased risk of CV disease (pooled odds ratio [OR] 1.43, 95% CI: 1.24–1.66)

55% increased risk of CV events (pooled OR 1.22–1.96)
How would you treat a patient like Mark who has comorbidities?
Help your eligible patients feel relief from the daily burden of PsA3–7
Real-world evidence shows a consistent safety profile over 9 years
No trend towards increased rates over time (pooled data; AS, PsA and PsO, in a PSUR including exposure in clinical trials and marketing experience).9
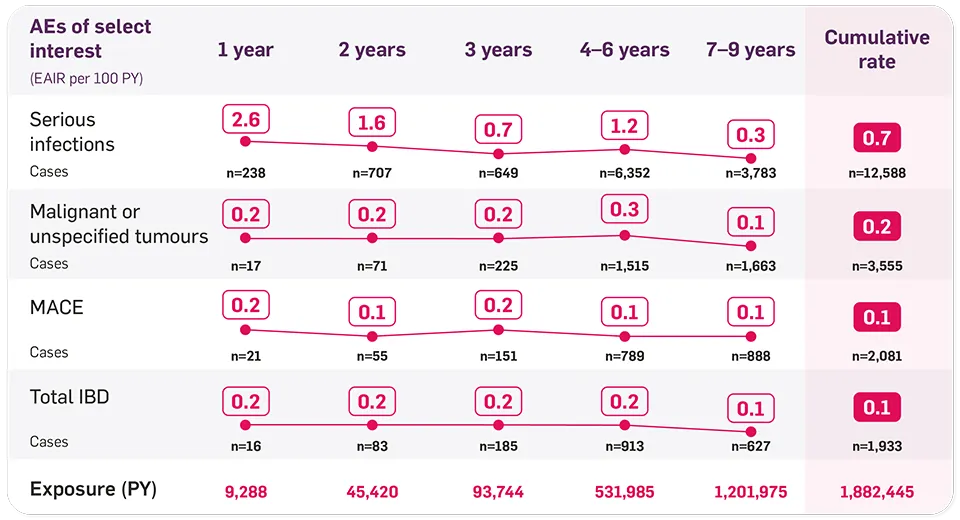
Source: Novartis Data on File, 2025.9
Successive time periods of PSUR shown with cumulative rate: 26 Dec 2014 to 25 June 2015; 26 June 2015 to 25 Dec 2015; 26 Dec 2015 to 25 June 2016; 26 June 2016 to 25 Dec 2016; 26 Dec 2016 to 25 Dec 2017; 26 Dec 2017 to 25 Dec 2018; 26 Dec 2018 to 25 Dec 2019; 26 Dec 2019 to 25 Dec 2020; 26 Dec 2020 to 25 Dec 2023.9
No trend toward increased rates of malignancy, MACE or IBD over time9
<1% incidence rate of IBD, which is within expected background incidence rate (per 100 PY)9,10
1,882,000+ PY across PsA, AS and PsO indications9
The most frequently reported adverse reactions with Cosentyx are upper respiratory tract infections (17.1%) (most frequently nasopharyngitis, rhinitis)1
Caution should be exercised when considering the use of Cosentyx in patients with a chronic infection or a history of recurrent infection. If a patient develops a serious infection, the patient should be closely monitored and Cosentyx should not be administered until the infection resolves.1
Patients should be evaluated for tuberculosis infection prior to initiating treatment with Cosentyx. Cosentyx should not be given to patients with active tuberculosis. In patients with latent tuberculosis, anti-tuberculosis therapy should be considered prior to initiation of Cosentyx.1
Cosentyx is not recommended in patients with IBD. If a patient develops signs and symptoms of IBD or experiences an exacerbation of pre-existing inflammatory bowel disease, Cosentyx should be discontinued, and appropriate medical management should be initiated.1
Refer to the SmPC for full safety information before prescribing.1

§Since first indication in 2015 for eligible patients with moderate to severe PsO.12
Do you consider comorbidities when managing patients with PsA?
Find out more about Cosentyx’s safety profile
Therapeutic indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active non-radiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*In a meta-analysis of observational studies comprising patients with PsA (n=32,973), compared with the general population.2
†ULTIMATE: Observed data in biologic-naïve patients with PsA receiving Cosentyx 300 mg or 150 mg who were originally randomly assigned to Cosentyx (n=83).8
‡FUTURE 2: Post-hoc analysis of observed data over 104 weeks for the Cosentyx 300 mg treatment group of biologic-naïve patients with this symptom at baseline, including those originally randomly assigned to Cosentyx and placebo-switchers (number of patients originally randomised to Cosentyx 300 mg, n=67; number of evaluable patients at Week 104, n=57).3
ACR, American College of Rheumatology; AE, adverse event; AS, ankylosing spondylitis; CI, confidence interval; CV, cardiovascular; EAIR, exposure-adjusted incidence rate; ERA, enthesitis-related arthritis; HS, hidradenitis suppurativa; IBD, inflammatory bowel disease; JPsA, juvenile psoriatic arthritis; MACE, major adverse cardiovascular event; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; OR, odds ratio; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSUR, periodic safety update report; PY, patient-year; SmPC, summary of product characteristics; TNFi, tumour necrosis factor inhibitor; VAS, visual analogue scale.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
Polachek A, et al. Arthritis Care Res 2017;69(1):67–74.
McInnes IB, et al. Arthritis Res Ther 2018;20(1):113.
Novartis Data on File. CAIN457F2342 (FUTURE 5): 2-year HAQ-DI biologic-naive data. February 2021.
Mease PJ, et al. Ann Rheum Did 2018;77:890–897.
Gossec L, et al. Ann Rheum Dis 2017;76(Suppl 2):949.
McInnes IB, et al. Lancet 2015;386(9999):1137–1146.
D'Agostino MA, et al. Semin Arthritis Rheum 2023;63:152259.
Novartis Data on File. Secukinumab (SEC020). April 2025.
Gottlieb AB, et al. Acta Derm Venereol 2022;102:adv00698.
Novartis Data on File. Secukinumab (SEC018). February 2025.
European Medicines Agency. Summary of positive opinion. EMA/CHMP/670627/2015. Available at: https://www.ema.europa.eu/en/documents/smop/chmp-post-authorisation-summary-positive-opinion-cosentyx_en.pdf [Accessed January 2026].
UK | January 2026 | FA-11538593
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.