Cosentyx® (secukinumab): Efficacy in psoriatic arthritis (PsA)
Cosentyx is indicated for the treatment of: moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adults (alone or in combination with methotrexate [MTX]) who have responded inadequately to disease-modifying anti-rheumatic drug therapy.1
Please refer to the Cosentyx Summary of Product Characteristics for full safety information, and the safety profile page here.
Are you using a treatment option recommended for all six key manifestations of PsA?
Cosentyx has demonstrated clinical efficacy, which was observed to last, in all six key manifestations of PsA2–6
Key clinical manifestations of PsA are joint, axial, skin, enthesitis, dactylitis and nails.3
Lasting: results observed at Week 52+.
Joints

68%
achieved ACR50
at Year 1 (n=44/65; observed data; secondary endpoint) in ULTIMATE*2
Axial
69%
sustained ASAS40
at Year 1 (n=96/139; observed data; secondary endpoint) in MAXIMISE†3
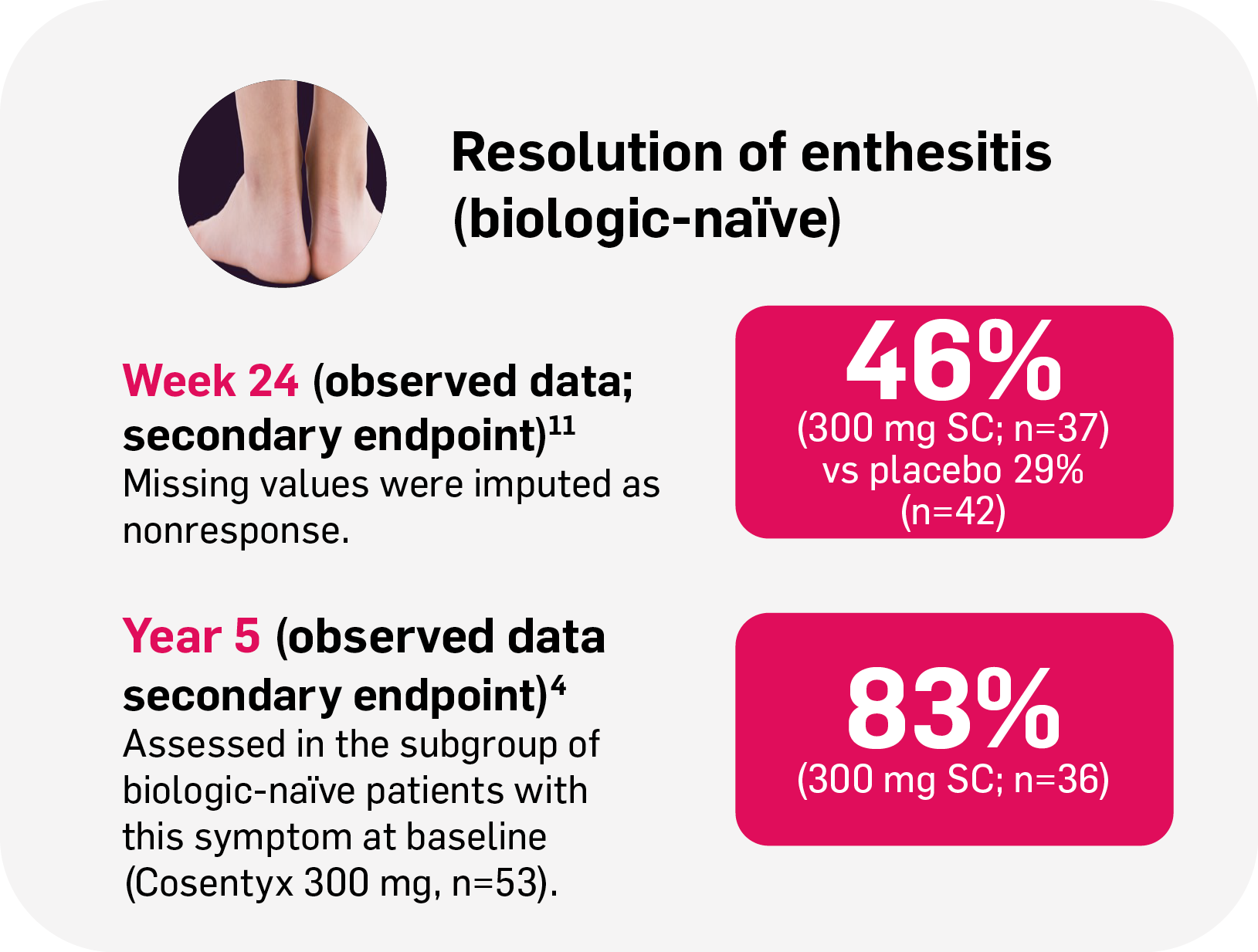
Enthesitis
76%
achieved resolution at Year 5 (n=39/51; observed data; secondary endpoint) in FUTURE 2‡4
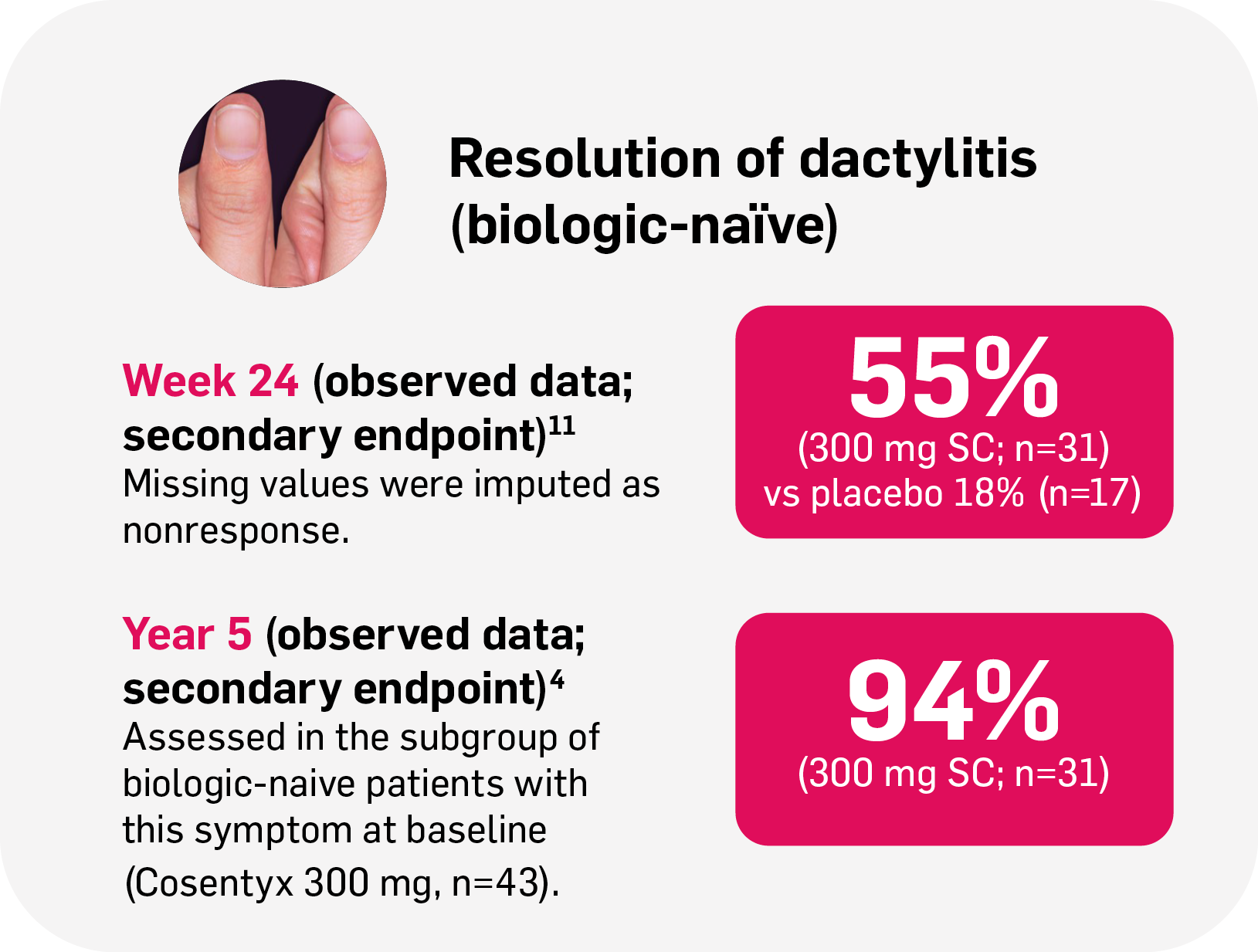
Dactylitis

88%
achieved resolution at Year 5 (n=35/40; observed data; secondary endpoint) in FUTURE 2§4
Nails
–73%
observed improvement from baseline in mNAPSI at 2.5 years (n=66; NRI; observed data) in TRANSFIGURE¶5
Skin
55%
achieved PASI 100 at Year 1 (n=23/41; observed data; secondary endpoint) in MATURE‖6
All the primary endpoints were met in the studies referenced above. Use the buttons below to navigate through more information.
FAST and LASTING relief from joint symptoms with Cosentyx2,7
FAST: ACR50 results seen as early as Week 2 in biologic-naïve Cosentyx-treated patients7
LASTING: ACR50 results observed to be maintained through to Year 12
ACR50 responders in biologic-naïve patients with PsA
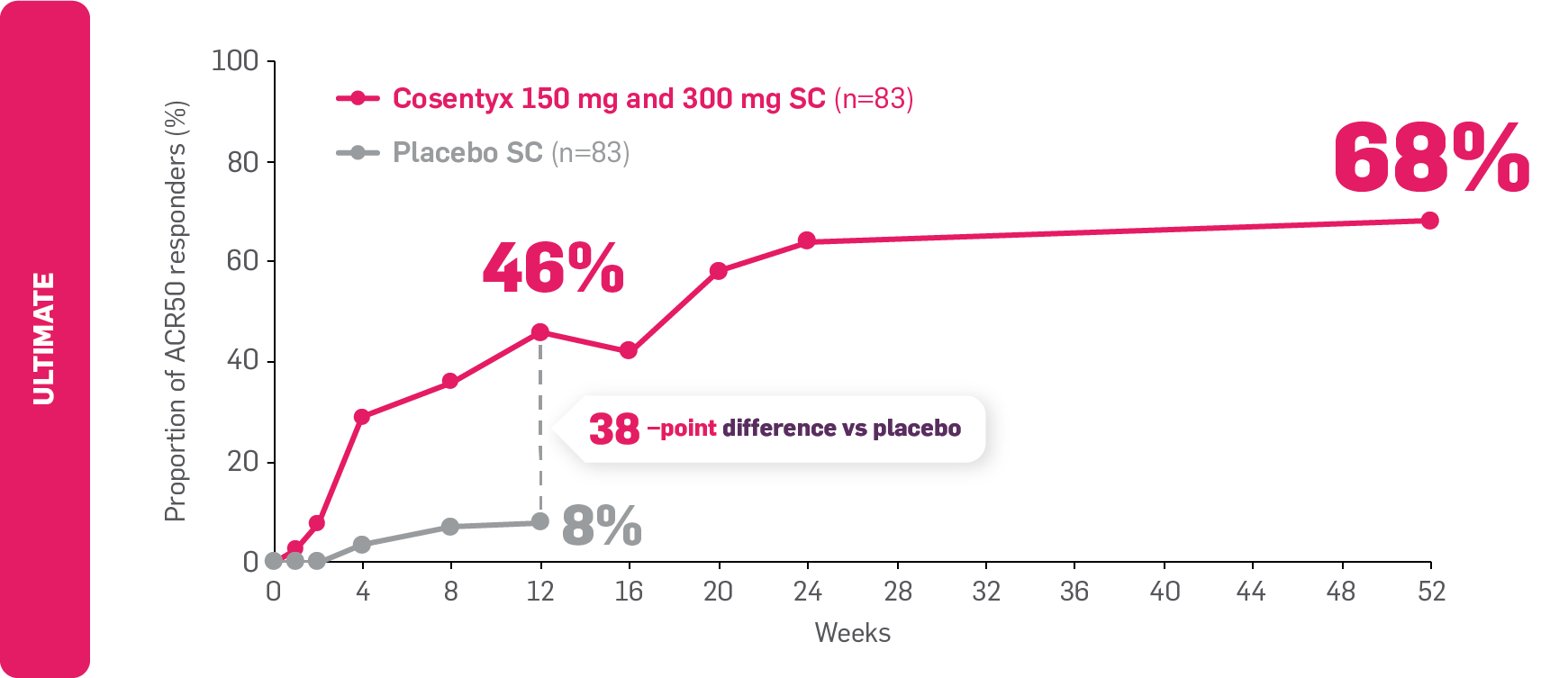
Adapted from D’Agostino MA, et al. 2023,2 and Boers M, et al. 2021.7
Data from Weeks 12 to 52 are as observed.2
ULTIMATE: The primary endpoint (GLOESS mean change from baseline at Week 12) was met (Cosentyx vs placebo: –9 vs –6, respectively; p=0.004).8
Data are based on non-responder imputation to Week 12 and are as observed from the open-label phase beyond this.7
Data from biologic-naïve patients through Week 24 are presented as NRI. Patients receiving Cosentyx 150 mg and 300 mg were pooled into a single arm. Patients received Cosentyx 150 mg if their BSA was ≤10%, or 300 mg if their BSA was >10% (as assessed by PASI score).7,8
FAST and LASTING joint symptom relief in TNFi-IR patients4,9
FAST: ACR50 results seen as early as Week 24 in TNFi-IR Cosentyx-treated patients with PsA9
LASTING: ACR50 results observed to be maintained through to Year 54
ACR50 responders in patients with PsA and IR or intolerance to TNFi
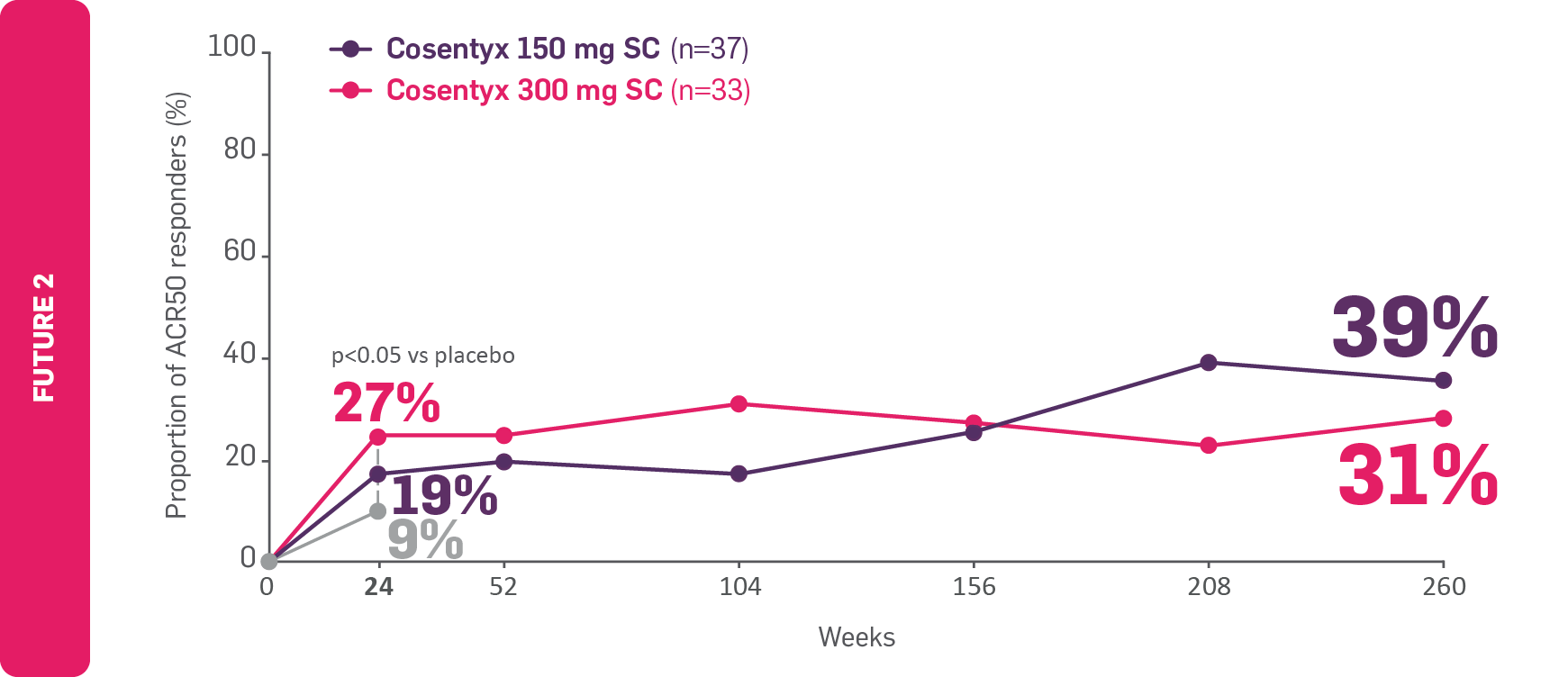
Adapted from McInnes IB, et al 20179 and McInnes IB, et al 2020.4
Data for Weeks 156, 208 and 260 are as observed.
FUTURE 2: The primary endpoint (the proportion of patients with ACR20 response at Week 24) was met (p<0.0001).9
For patients who are TNFi-IR, the recommended dose of Cosentyx is 300 mg SC with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Each 300 mg dose is given as one SC injection of 300 mg or two SC injections of 150 mg. For other patients, the recommended dose is 150 mg SC with initial dosing at Weeks 0, 1, 2, 3 and 4, followed by monthly maintenance dosing. Based on clinical response, the dose can be increased to 300 mg.1
P value at Week 24 derived from a logistic regression model with treatment as the factor and baseline weight as a covariate In the long-term extension of FUTURE 2, missing data for Week 52 were imputed as nonresponse.9
Data for Week 104 are presented with multiple imputation applied to missing variables.9 Data for Weeks 156, 208 and 260 are shown as observed in patients originally randomly assigned to their respective treatment groups.4 The Cosentyx 150 mg group included 29 patients in the biologic-naïve subgroup who were originally assigned to Cosentyx 150 mg and escalated to 300 mg starting at Week 128 or later, following a protocol amendment.4
Are you considering an appropriate treatment option for your patients’ individual needs?
FAST and LASTING skin clearance with Cosentyx6
FAST: PASI 100 results observed as early as Week 12 in patients with PsO6
LASTING: PASI 100 results observed to be maintained through to Week 52 (secondary endpoint; no comparator arm)6
PASI 100 responders in patients with PsO
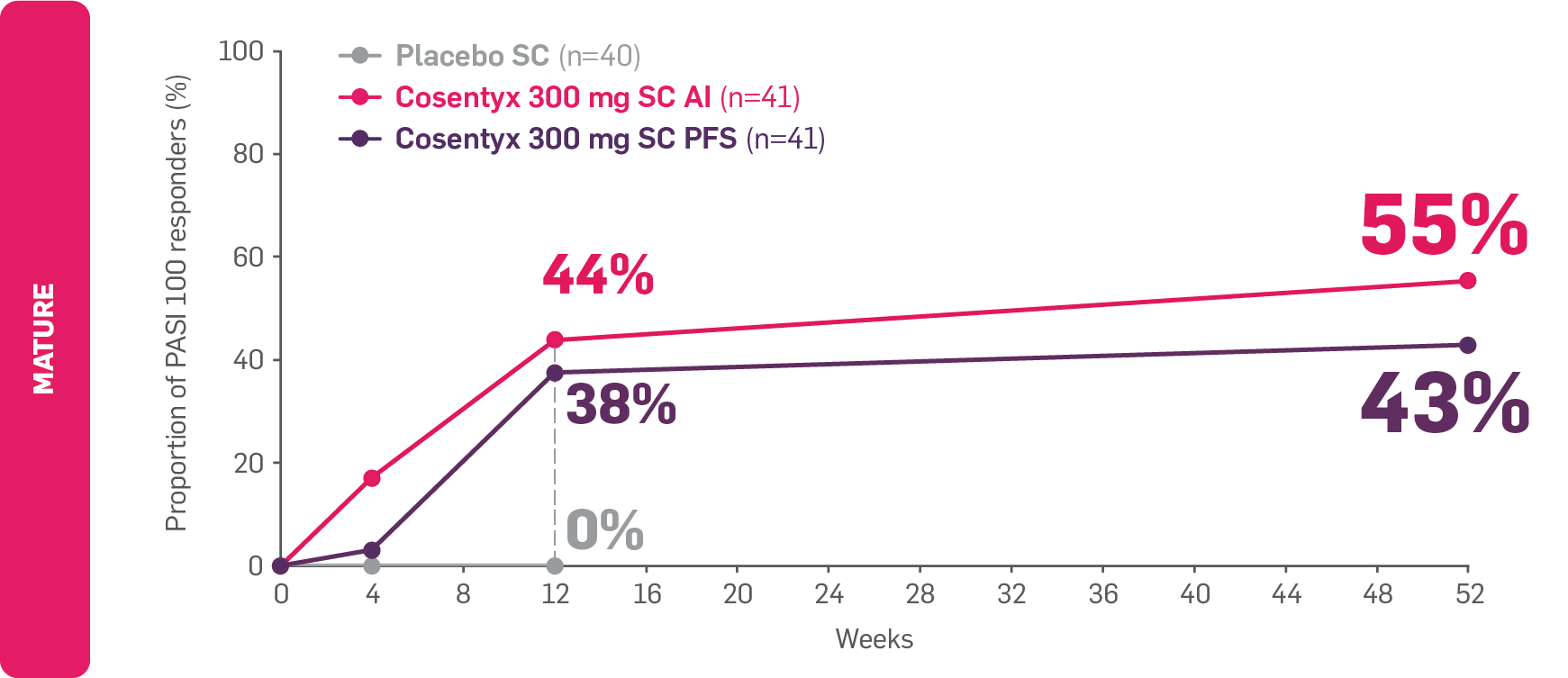
Adapted from Sigurgeirsson B, et al. 2022.6
Data from Weeks 12 to 52 are as observed.6
MATURE: The co-primary endpoints (PASI 75 and IGA 0/1 at Week 12) were met (PASI 75: 95.1%, 83.2% and 10% for Cosentyx 300 mg AI, PFS and placebo, respectively; IGA 0/1: 75.6%, 68.1% and 7.6% for Cosentyx 300 mg AI, PFS and placebo, respectively; p<0.0001).6
At baseline, approximately 16% of patients with moderate to severe PsO had previously received a biologic therapy.6
Do your patients need a more individualised treatment approach for both skin and joints?
FAST and LASTING relief from axial symptoms with Cosentyx3
FAST: ASAS40 results seen as early as Week 12 in biologic-naïve patients.3
LASTING: ASAS40 results observed to be maintained through to Week 52.3
ASAS40 responders in biologic-naïve patients with axial PsA
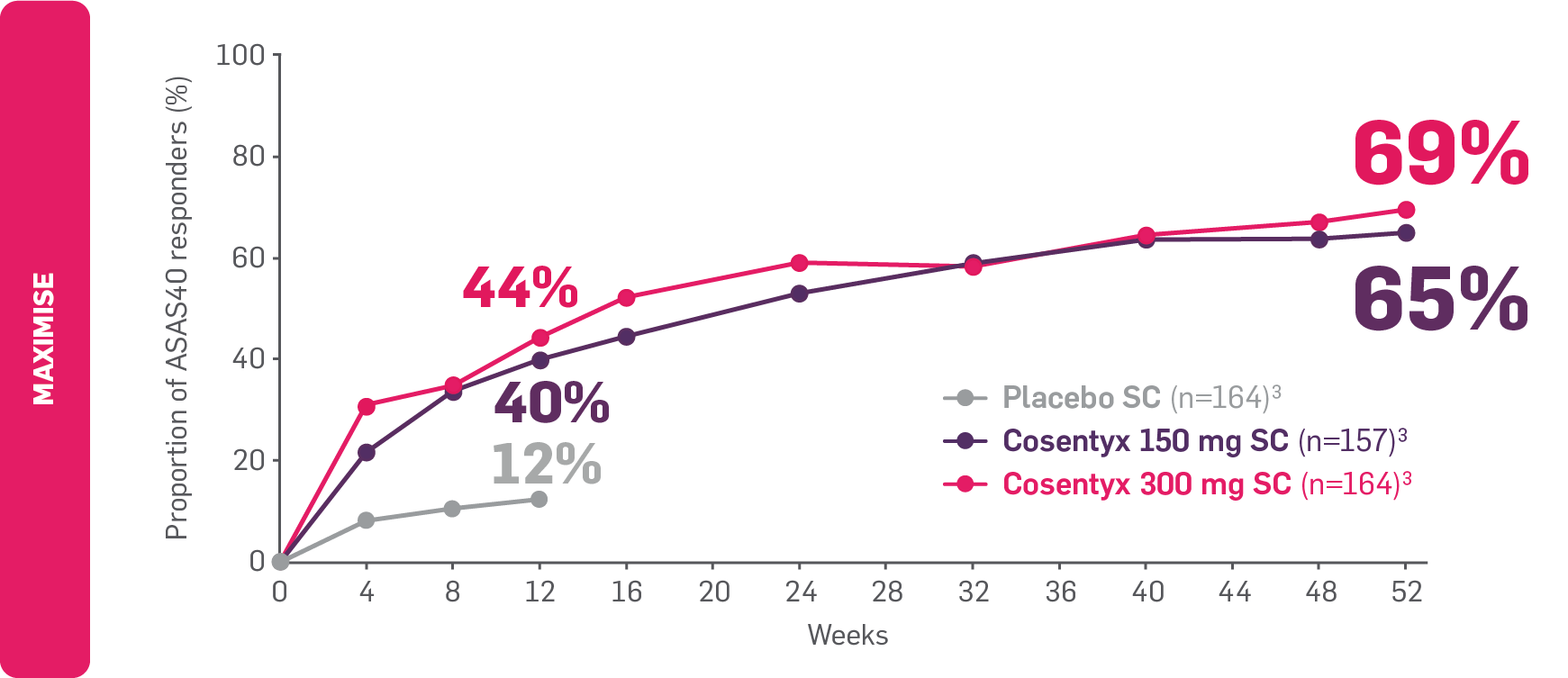
Adapted from Novartis Data on File. 2020.10
Data from Weeks 12 to 52 are as observed.3
MAXIMISE: The primary endpoint (ASAS20 response at Week 12) was met (63% vs 31% for Cosentyx 300 mg vs placebo, respectively; p<0.0001).3
ASAS20 and ASAS40 were pre-specified exploratory endpoints at Week 12. No clinical or statistical conclusions can be drawn. 83% and 86% of patients completed the trial in the 300 mg and 150 mg groups, respectively.3
Find out more about Cosentyx in eligible patients with axSpA
Resolution of enthesitis and dactylitis with Cosentyx4,11
FUTURE 2: The primary endpoint (proportion of patients with ACR20 response at Week 24) was met (54%, 51% and 15% for Cosentyx 300 mg, 150 mg and placebo, respectively; p<0.0001).12
FAST and LASTING nail symptom relief with Cosentyx5,13
FAST: NAPSI results seen as early as Week 16 in patients with nail psoriasis.13
LASTING: NAPSI results observed to be maintained through to 2.5 years.5
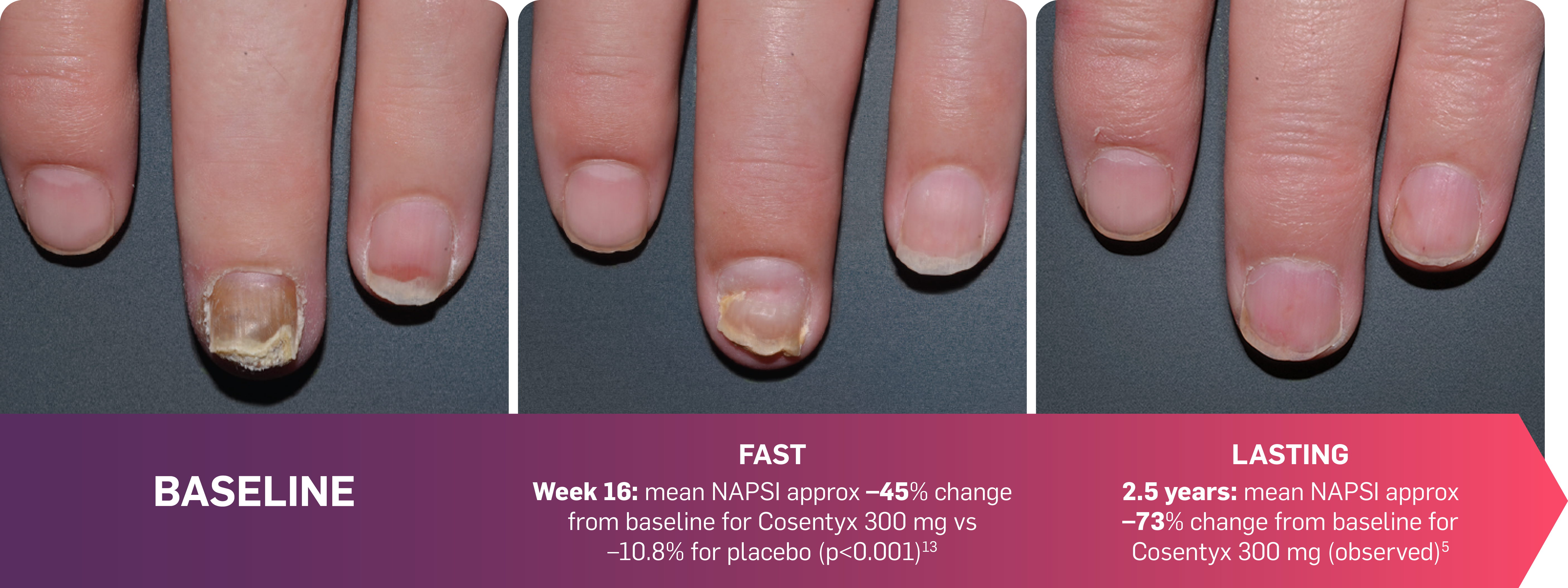
Actual photos taken of a Cosentyx patient by investigators during clinical trials. Individual patient responses may vary.
TRANSFIGURE: The primary endpoint (percentage change from baseline in mean NAPSI score at Week 16) was met (p<0.001).13
FAST scalp clearance was observed through to Week 24 with Cosentyx14
FAST: PSSI results seen as early as Week 12 in patients with scalp psoriasis.14
Scalp clearance refers to a PSSI 90 response.14
Adapted from Bagel J, et al. 2017.14
Baseline PSSI score: 33.4 (Cosentyx 300 mg; n=51)
The primary endpoint (proportion of patients achieving PSSI 90 at Week 12) was met (53% vs 2% for Cosentyx 300 mg and placebo, respectively; p<0.001).14
LASTING retention observed to Year 5 with Cosentyx15
Lasting retention: number of patients who responded to Cosentyx and stayed with Cosentyx to Year 5.15
SERENA is the largest prospective, longitudinal, non-interventional study with Cosentyx in adult patients with moderate to severe PsO, active PsA or active AS.16,17
The primary objective was to assess the long-term retention of Cosentyx in routine clinical practice. Secondary objectives of the study were to describe the long-term effectiveness of Cosentyx for the treatment of PsA and AS.16

>2,900 patients
treated with Cosentyx16

19 countries16

438 sites
across Europe (including the UK), Israel and Russia16,17
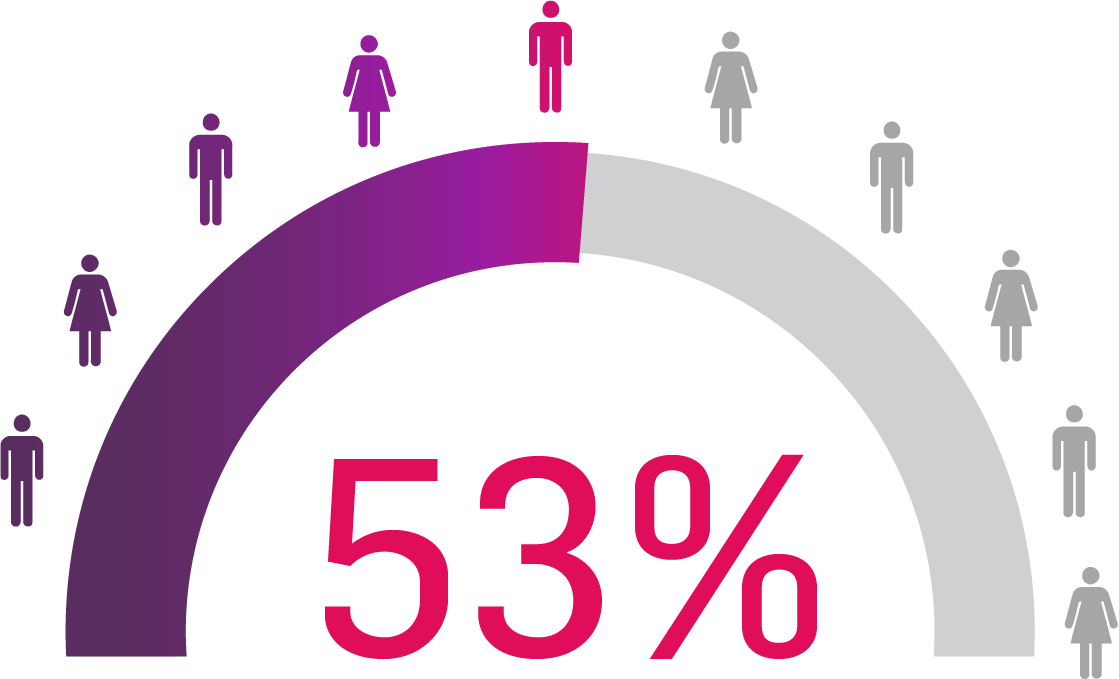
56% of patients who responded to Cosentyx, stayed on Cosentyx15
SERENA real-world data shows that 56% of patients with PsA who responded to Cosentyx* stayed on Cosentyx to Year 5 (n=522)15
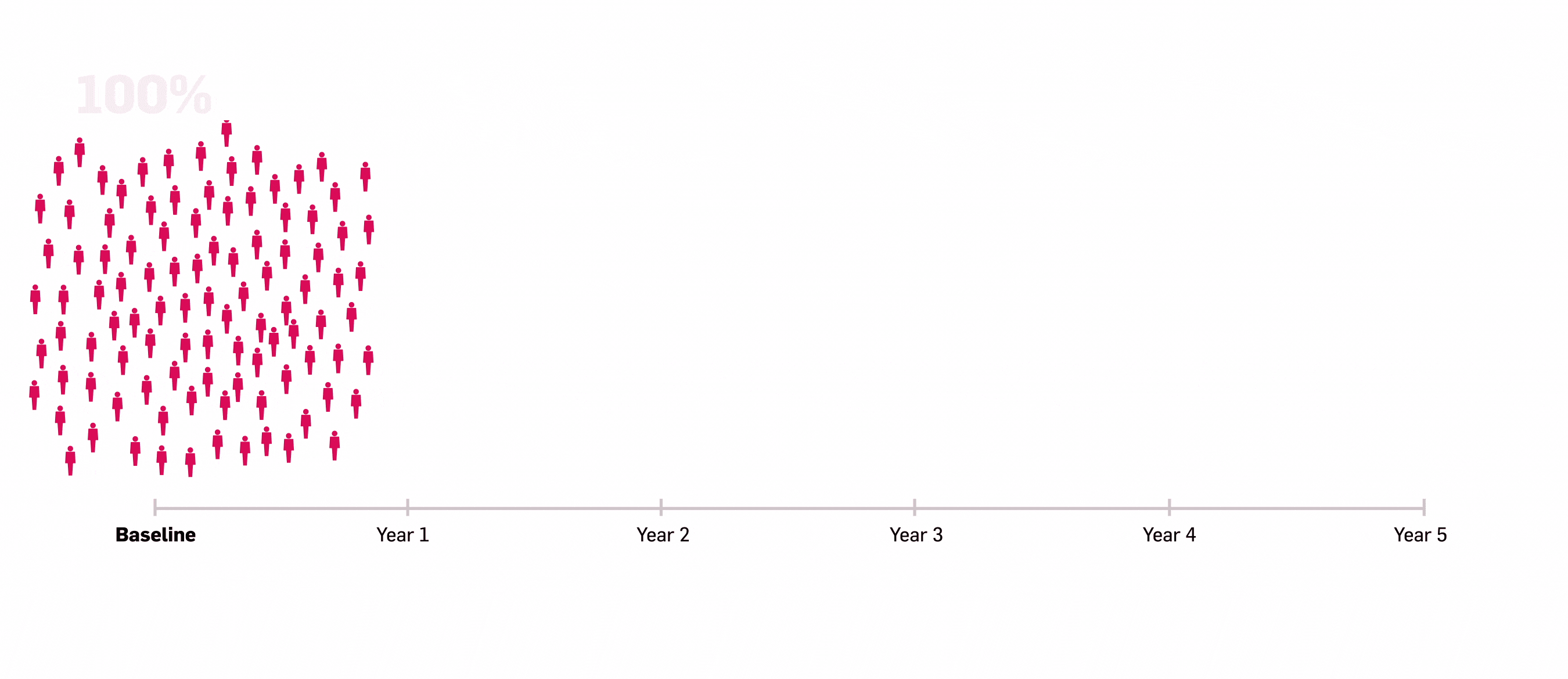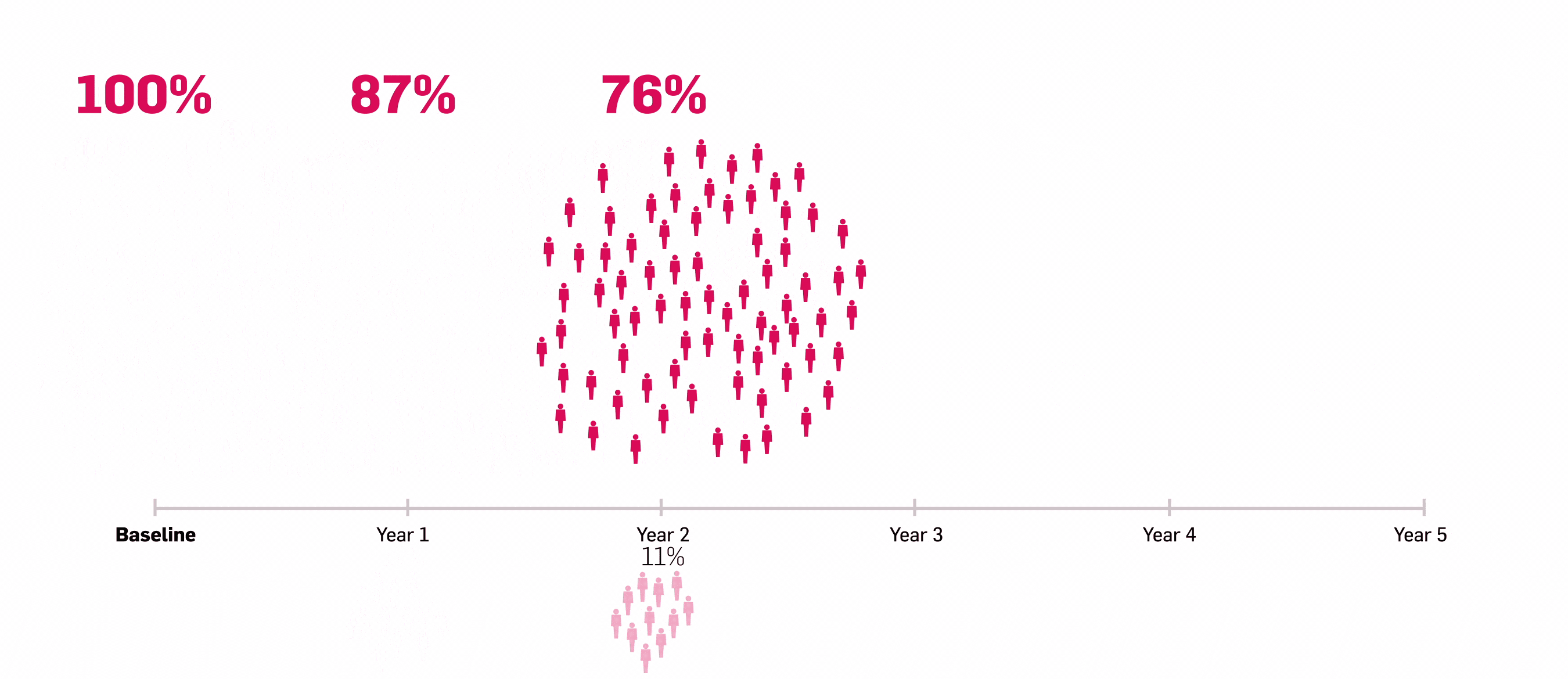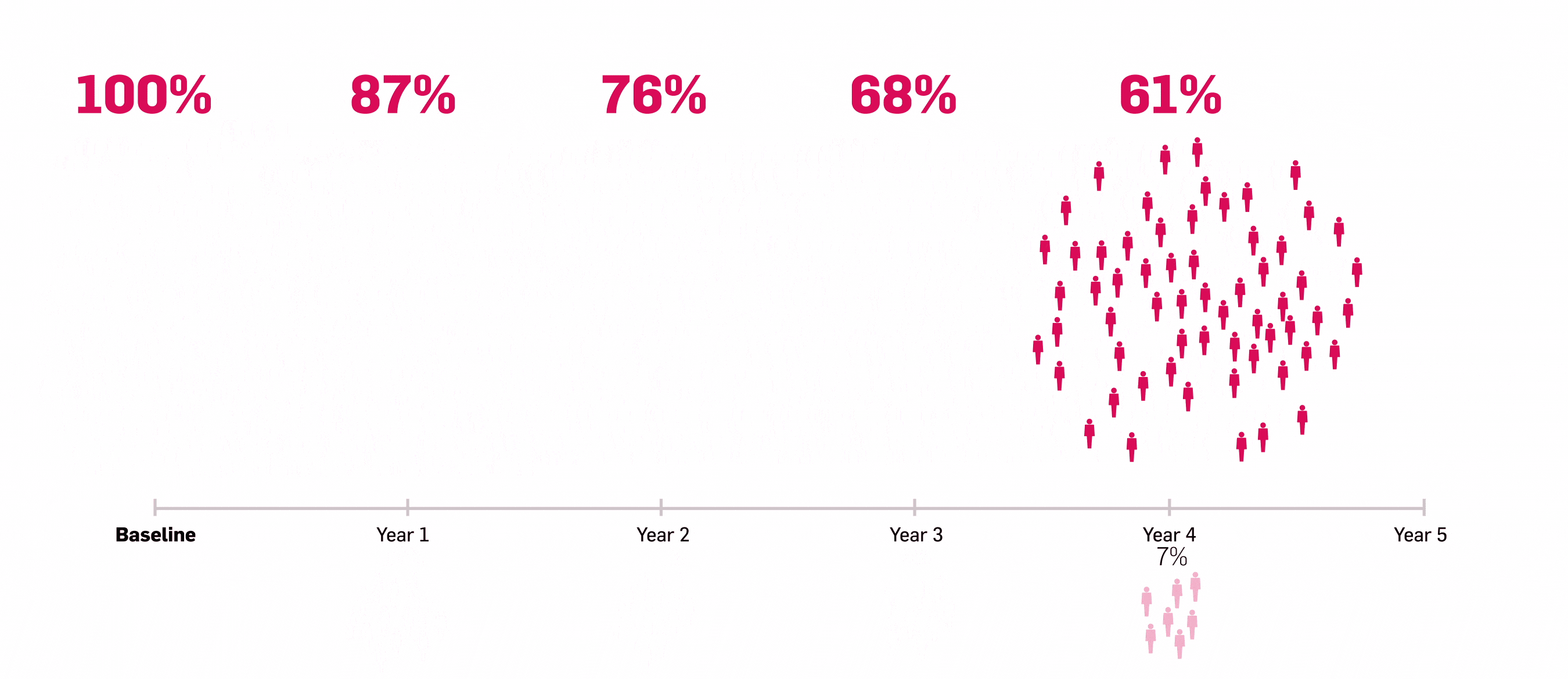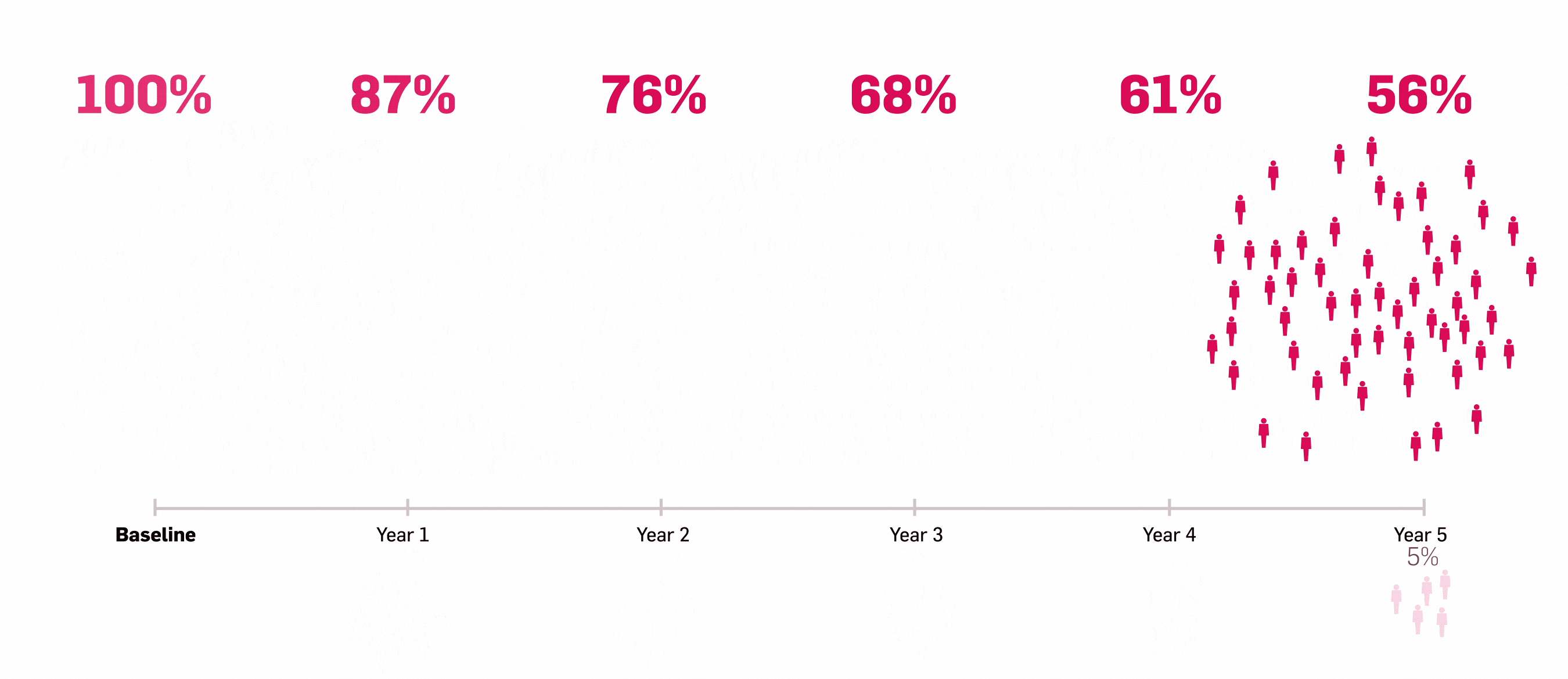
Based on Kiltz U, et al. 2024.15
Retention rates were analysed using Kaplan–Meier.
*The study included patients aged ≥18 years who had received ≥16 weeks of treatment before enrollment.15
Could remission be achievable for your eligible patients with PsA? Learn more about MDA and Cosentyx, and what this could mean for your eligible patients
Therapeutic Indications1
Cosentyx is indicated for the treatment of moderate to severe plaque psoriasis (PsO) in adults, children and adolescents from the age of 6 years who are candidates for systemic therapy; active psoriatic arthritis (PsA) in adult patients (alone or in combination with methotrexate [MTX]) when the response to previous disease-modifying anti-rheumatic drug therapy has been inadequate; active ankylosing spondylitis (AS) in adults who have responded inadequately to conventional therapy; active nonradiographic axial spondyloarthritis (nr-axSpA) with objective signs of inflammation as indicated by elevated C-reactive protein and/or magnetic resonance imaging evidence in adults who have responded inadequately to non-steroidal anti-inflammatory drugs; active moderate to severe hidradenitis suppurativa (HS; acne inversa) in adults with an inadequate response to conventional systemic HS therapy; active enthesitis-related arthritis (ERA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy; active juvenile psoriatic arthritis (JPsA) in patients 6 years and older (alone or in combination with MTX) whose disease has responded inadequately to, or who cannot tolerate, conventional therapy.1
*ULTIMATE: Observed data in biologic-naïve patients with PsA receiving Cosentyx 300 mg or 150 mg who were originally randomly assigned to Cosentyx (n=83).2
†MAXIMISE: Observed data in biologic-naïve patients with PsA in the Cosentyx 300 mg treatment group (n=139); in the respective Cosentyx 150 mg treatment group, 65% achieved ASAS40 at Year 1 (n=141).3
‡FUTURE 2: Observed data for the Cosentyx 300 mg treatment group of biologic-naïve patients with this symptom at baseline, including those originally randomly assigned to Cosentyx and placebo-switchers (n=36); 76% in the respective Cosentyx 150 mg group maintained complete resolution of enthesitis through Year 5 (n=45).4
§FUTURE 2: Observed data for the Cosentyx 300 mg treatment group of biologic-naïve patients with this symptom at baseline, including those originally randomly assigned to Cosentyx and placebo-switchers (n=31); 86% in the respective Cosentyx 150 mg group maintained complete resolution of dactylitis through Year 5 (n=22).4
¶MATURE: Percentage of patients with moderate-to-severe PsO at baseline by visit (multiple imputation) for patients receiving Cosentyx 300 mg by autoinjector (n=41); in the Cosentyx 300 mg prefilled syringe group (n=41), 43% achieved PASI 100 at Year 1.6
IITRANSFIGURE: Observed data in patients with moderate to severe nail PsO in the Cosentyx 300 mg treatment group (n=66; NRI; observed data).5
ACR, American College of Rheumatology; AI, autoinjector; AS, ankylosing spondylitis; ASAS, Assessment of Spondyloarthritis international Society; axSpA, axial spondyloarthritis; BSA, body surface area; ERA, enthesitis-related arthritis; EULAR, European Alliance of Associations for Rheumatology; GLOESS, Global OMERACT-EULAR synovitis score; HS, hidradenitis suppurativa; IGA, investigator’s global assessment; IR, inadequate responder; JIA, juvenile idiopathic arthritis; JPsA, juvenile psoriatic arthritis; mNAPSI, modified nail psoriasis severity index; MTX, methotrexate; nr-axSpA, non-radiographic axial spondyloarthritis; NRI, non-responder imputation; OMERACT, outcome measures in rheumatology; PASI, psoriasis area and severity index; PFS, pre-filled syringe; PsA, psoriatic arthritis; PsO, plaque psoriasis; PSSI, psoriasis scalp severity index; SC, subcutaneous; SmPC, summary of product characteristics; TNFi-IR, tumour necrosis factor inhibitor-inadequate responder.
References
Cosentyx® (secukinumab) Summary of Product Characteristics.
D’Agostino MA, et al. Semin Arthritis Rheum 2023;63:152259.
Baraliakos X, et al. Ann Rheum Dis 2021;80(5):582–590.
McInnes IB, et al. Lancet Rheumatol 2020;2(4):e227–e235 and supplementary appendix.
Reich K, et al. Br J Dermatol 2021;184(3):425–436.
Sigurgeirsson B, et al. Dermatol Ther 2022;35(3):e15285.
Boers M, et al. Poster POS0197B. EULAR European Congress of Rheumatology. 2–5 June 2021; virtual.
D’Agostino MA, et al. Rheumatology (Oxford). 2022;61(5):1867–1876 and supplementary appendix.
McInnes IB, et al. Rheumatology (Oxford). 2017;56(11):1993–2003.
Novartis Data on File. CAIN457F3302 (MAXIMISE) Clinical Study Report. August 2020.
Kavanaugh A, et al. J Rheumatol 2016;43(9):1713–1717.
McInnes IB, et al. Lancet 2015;386(9999):1137–1146.
Reich K, et al. Br J Dermatol 2019;181(5):954–966.
Bagel J, et al. J Am Acad Dermatol 2017;77:667–674.
Kiltz U et al. Abstract 1840456. American College of Rheumatology (ACR) Convergence, 14–19 November 2024; Washington, DC, US.
Kiltz U, et al. Adv Ther 2020;37(6):2865–2883.
Kiltz U, et al. Rheumatol Ther 2022;9(4):1129–1142.
UK | July 2025 | FA-11426985-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.