Contact us
Need more information? Your local key account manager will be in contact shortly.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features.1
These patient case studies are based on real UK patients and are designed to help identify potential eligible patients who might benefit from KESIMPTA treatment. Please note that the patient images are not of real patients. The patient descriptions are based on real patient case studies of someone with active relapsing-remitting multiple sclerosis (RRMS).
Individual experiences with KESIMPTA may vary; please refer to the Summary of Product Characteristics (SmPC) for typical responses.
Cases below should not be read in isolation. For full prescribing information, please refer to the KESIMPTA Summary of Product Characteristics (SmPC).1
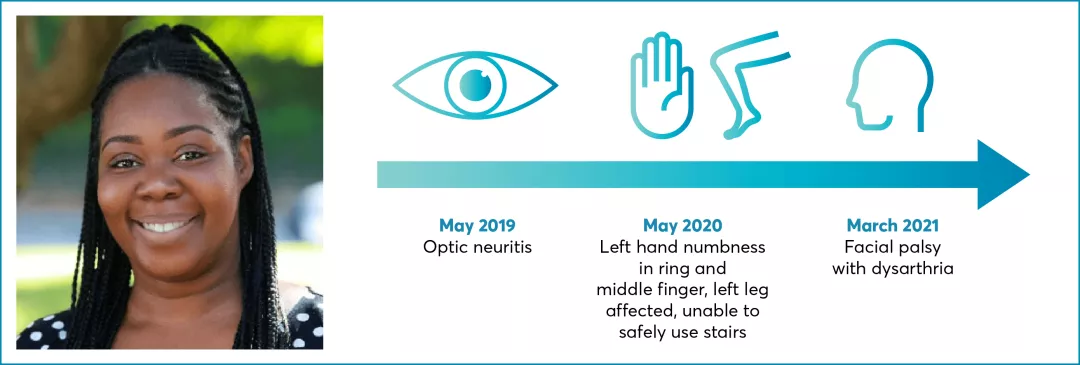
RRMS with active disease defined by clinical/imaging features
EDSS 1.0
Sibling with MS
No comorbidities
Family complete
Women of childbearing potential should use effective contraception (methods that result in less than 1% pregnancy rates) while receiving KESIMPTA and for 6 months after the last administration of KESIMPTA.1
KESIMPTA met the primary endpoint in ASCLEPIOS I/II, with up to 58% reduction in ARR vs teriflunomide (ASCLEPIOS I: 51% [0.11 vs 0.22]; RR 0.49 [95% CI: 0.37–0.65]; ASCLEPIOS II: 58% [0.10 vs 0.25]; RR 0.42 [95% CI: 0.31–0.56]; both p<0.001).3
Data from exploratory/post-hoc analyses are descriptive and no confirmatory clinical conclusions can be drawn.
KESIMPTA was associated with a transient decrease of 4.3% in mean IgG levels after 48 weeks of treatment but an increase of 2.2% after 96 weeks.1 Post-hoc analysis showed mean serum IgG levels remained stable up to 6 years of treatment, and the majority of patients (97.2%) had IgG levels above the LLN (N=1969); data cut-off: September 2023.2 In the Phase III clinical studies, a decrease in mean value of IgM (30.9% decrease after 48 weeks and 38.8% decrease after 96 weeks) was observed and no association with risk of infections, including serious infections, was shown (N=1969); data cut-off: September 2023.1,2 Post-hoc analysis showed mean IgM levels decreased but remained above the LLN up to 6 years in the majority of patients (65.9%).2 The most important and frequently reported adverse reactions are upper respiratory tract infections (39.4%), systemic injection-related reactions (20.6%), injection-site reactions (10.9%) and urinary tract infections (11.9%).1
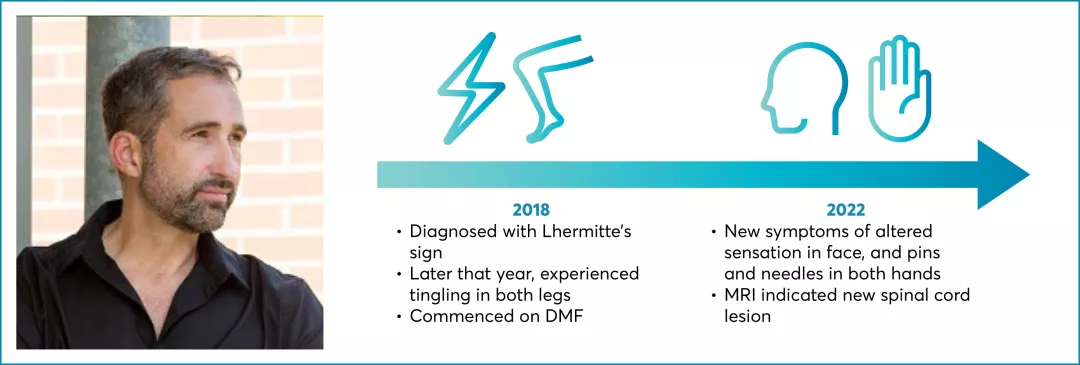
RRMS with active disease defined by clinical/imaging features
EDSS 2.5
KESIMPTA is contraindicated in patients with a hypersensitivity to the active substance or to any of the excipients listed in the SmPC, patients in a severely compromised state, with severe active infection or known active malignancy.1
Since John Cunningham virus infection resulting in progressive multifocal leukoencephalopathy (PML) has been observed in patients treated with anti-CD20 antibodies, other MS therapies, and KESIMPTA at substantially higher doses in oncology indications, physicians should be vigilant for medical history of PML and for any clinical symptoms or MRI findings that may be suggestive of PML. If PML is suspected, treatment with KESIMPTA should be suspended until PML has been excluded.1
Hepatitis B reactivation has occurred in patients treated with anti-CD20 antibodies, which in some cases resulted in fulminant hepatitis, hepatic failure and death.
Patients with active hepatitis B disease should not be treated with KESIMPTA. Hepatitis B virus (HBV) screening should be performed in all patients before initiation of treatment. As a minimum, screening should include hepatitis B surface antigen (HBsAg) and hepatitis B core antibody (HBcAb) testing. These can be complemented with other appropriate markers as per local guidelines. Patients with positive hepatitis B serology (either HBsAg or HBcAb) should consult a liver disease expert before the start of treatment and should be monitored and managed following local medical standards to prevent hepatitis B reactivation.1
It is recommended to evaluate the patient‘s immune status prior to initiating therapy.
Based on its mode of action and available clinical experience, KESIMPTA has the potential for an increased risk of infections. Administration should be delayed in patients with an active infection until the infection is resolved. KESIMPTA must not be given to patients in a severely immunocompromised state (e.g. significant neutropenia or lymphopenia).1
Please refer to the KESIMPTA SmPC for full safety information.1
For full prescribing information, please refer to the KESIMPTA Summary of Product Characteristics (SmPC).1
*KESIMPTA has demonstrated efficacy in ASCLEPIOS I and II (2-year core studies), two Phase III randomised, double-blind, double-dummy, active comparator-controlled, parallel-group, multicentre pivotal trials, comparing a range of outcomes to active comparator teriflunomide in 1882 patients with relapsing MS, following patients for up to 6 years (including the 4-year extension phase, ALITHIOS study).1–3 ALITHIOS was an ongoing, open-label, single-arm, umbrella-extension, Phase IIIb study assessing the risk–benefit profile of KESIMPTA (20 mg subcutaneously every 4 weeks) and its tolerability in patients with RMS. The study enrolled 1703 patients with RMS from the APLIOS, APOLITOS and ASCLEPIOS I/II trials who continued KESIMPTA treatment.2,5
†NEDA-3 is defined as no 6-month confirmed disability worsening, no confirmed MS relapse, no new or enlarging T2 lesions and no T1 Gd+ lesions.2
‡ARR was defined as the number of confirmed MS relapses per year, according to prespecified criteria. Confirmed relapses are those accompanied by a clinically relevant change in EDSS.2,3
§Based on a cross-sectional survey of adult patients with RMS (N=105) in the US who self-administered KESIMPTA with the KESIMPTA pen within the previous 12 months. A total of seven attributes of the KESIMPTA pen were assessed: ‘administration time’, ‘overall ease of use’, ‘easy of monthly dosing schedule’, ‘time required to device preparation’, ‘ease of device preparation’, ‘device ergonomics’ and ‘portability’.4
ARR, annualised relapse rate; CI, confidence interval; DMT, disease-modifying therapy; EDSS, expanded disability status score; Gd+, gadolinium; HBV, hepatitis B virus; IgG, immunoglobulin G; IgM, immunoglobulin M; LLN, lower limit of normal; MRI, magnetic resonance imaging; MS, multiple sclerosis; NEDA-3, no evidence of disease activity; PML, progressive multifocal leukoencephalopathy; RMS, relapsing forms of MS; RRMS, relapsing-remitting multiple sclerosis; SmPC, Summary of Product Characteristics.
References
KESIMPTA® (ofatumumab) Summary of Product Characteristics.
Wiendl H, et al. Poster P9.010. Academy of Neurology (AAN) Annual Meeting, 13–18 April 2024, Denver, US.
Hauser SL, et al. New Engl J Med 2020;383(6):546–557.
Ross AP, et al. Poster LB09. Consortium of Multiple Sclerosis Centers (CMSC) Annual Meeting, 31 May–3 June 2023.
Hauser SL, et al. Mult Scler 2023;29(11–12):1452–1464.
UK | July 2025 | FA-11353853
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.