Prescribing information (external link)


KESIMPTA®▼(ofatumumab) resources
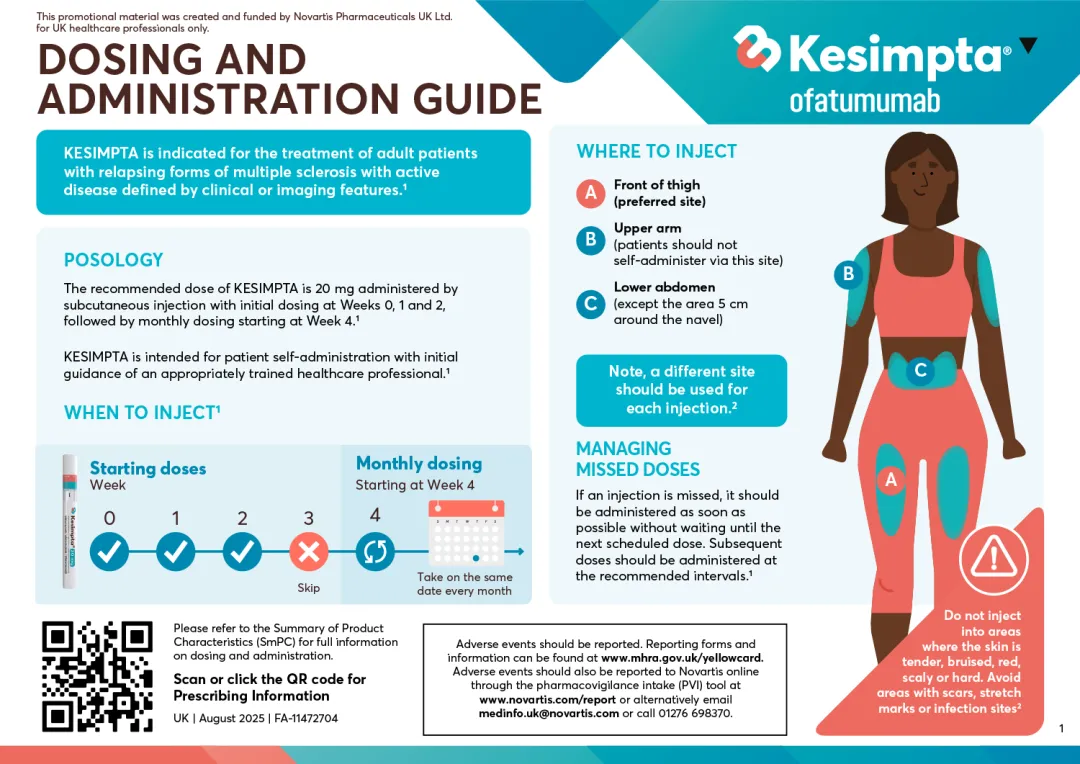
KESIMPTA is indicated for the treatment of adult patients with relapsing forms of multiple sclerosis (RMS) with active disease defined by clinical or imaging features.1
This selection of resources is designed for healthcare professionals (HCPs) to support patients with RMS throughout their KESIMPTA journey.
Below you can find helpful resources for you and your patients. Please note that only the resources in the ‘Resources for patients’ section can be downloaded and shared with your KESIMPTA patients. Please do not share this web page with patients; instead, please direct them to the patient portal where they can access these resources.
HCP, healthcare professional; RMS, relapsing forms of multiple sclerosis.
Reference
KESIMPTA (ofatumumab) Summary of Product Characteristics.
UK | January 2026 | FA-11583610
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.