MPN10 Tracker
Help patients monitor symptoms with the MPN10 tracker.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
This link will take you to the electronic medicines compendium (emc) website, which is a non-Novartis website.
JAKAVI® (ruxolitinib) is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. JAKAVI® is also indicated for adult patients with polycythaemia vera who are resistant to or intolerant of hydroxyurea.1
This website is intended for healthcare professionals. Please do not direct patients to this page. Patient resources should be downloaded and forwarded to your patients. Alternatively, you can request hard copies from your local representative.
These resources have been developed by Novartis and are intended for healthcare professionals, to help you get the most out of treating with JAKAVI®.
JAKAVI dosing timeline
A JAKAVI® treatment timeline that can be used during your patient consultations.
MF risk stratification
A guide to support MF patient risk stratification using the IPSS, DIPSS and DIPSS Plus scoring systems.
A guide to JAKAVI in PV
A guide to the treatment with JAKAVI® for your patients with PV.
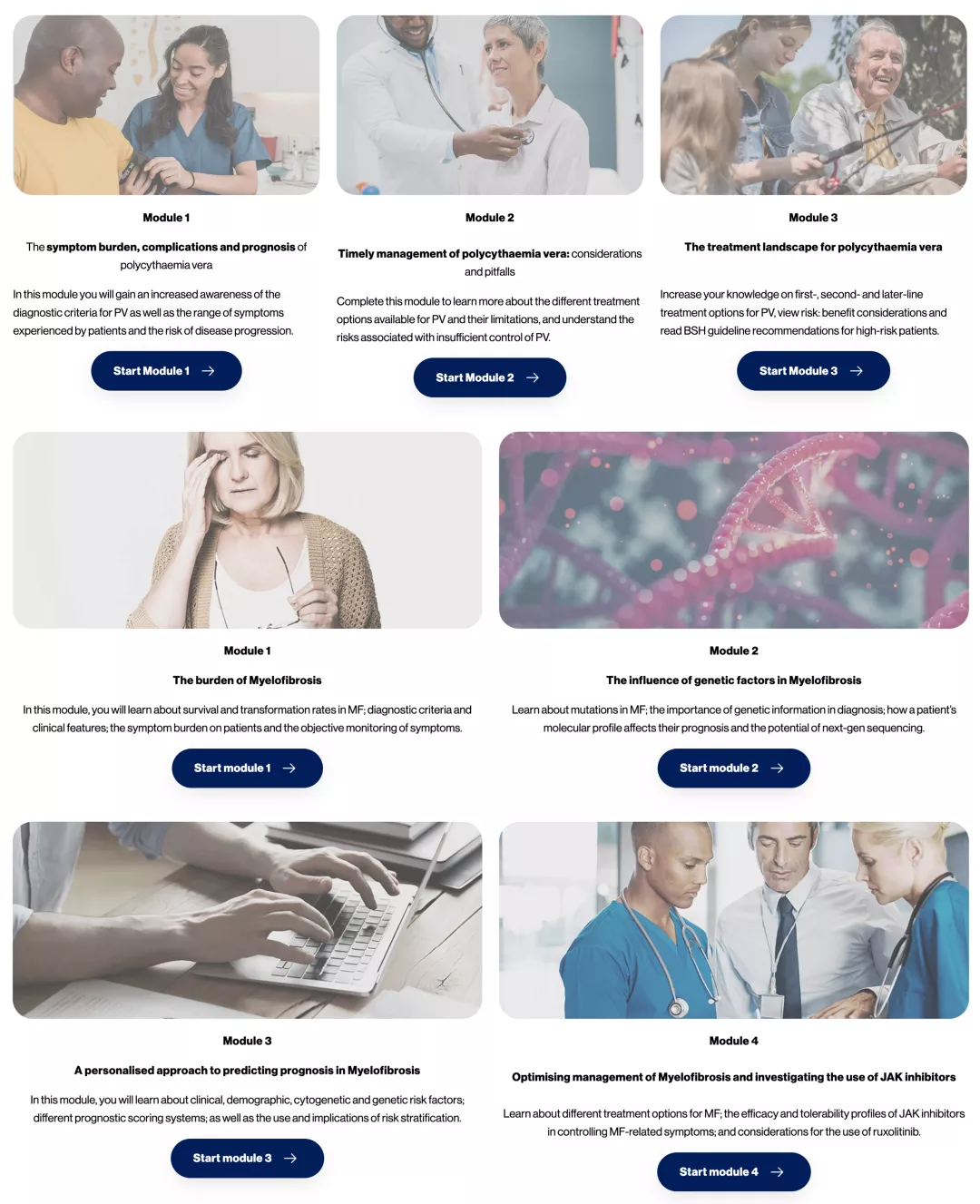
MF and PV educational modules
CPD-accredited modules on the treatment landscape, disease burden and management of MF and PV.
These resources have been developed by Novartis and are intended for patients who have been diagnosed with an MPN. These resources will help give patients the confidence and knowledge to discuss their disease and track symptoms effectively. Information provided in these resources should not be considered an alternative to advice from a healthcare professional. Please do not direct patients to this page. Patient resources should be downloaded and forwarded to your patients. Alternatively, you can request hard copies from your local representative.
Patient symptom guide
A guide to help patients identify and better understand symptoms related to their disease.
MF essentials guide
A guide to support patients with MF who have been initiated on JAKAVI®.
PV essentials guide
A guide to support patients with PV who have been initiated on JAKAVI®.
DIPSS, dynamic international prognostic scoring system; HCP, healthcare professional; IPSS, international prognostic scoring system; MF, myelofibrosis; MPN, myeloproliferative neoplasm; PV, polycythaemia vera.
Reference
JAKAVI® (ruxolitinib) Summary of Product Characteristics.
UK | November 2025 | FA-11317792
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.