Resources
Explore our range of resources which have been designed to support you in your management of patients.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
JAKAVI® (ruxolitinib) is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. JAKAVI® is also indicated for adult patients with polycythaemia vera who are resistant to or intolerant of hydroxyurea.1
For full safety information, please refer to the Summary of Product Characteristics.
Study | Type of study | Regimen | Study purpose and description | Study population |
|---|---|---|---|---|
COMFORT‑I2 | Phase III, double-blind, randomised controlled trial (RCT) | JAKAVI® (n=155) vs placebo (n=154) | Assessment of the efficacy and safety profile of JAKAVI® | Patients with intermediate-2 (int-2) or high-risk myelofibrosis (MF) |
COMFORT‑II3 | Phase III, open-label RCT | JAKAVI® (n=146) vs BAT (n=73) | Assessment of the efficacy and safety profile of JAKAVI® | Patients with int-2 or high-risk MF, post-polycythaemia vera (PV) MF or post-essential thrombocythaemia (ET) MF |
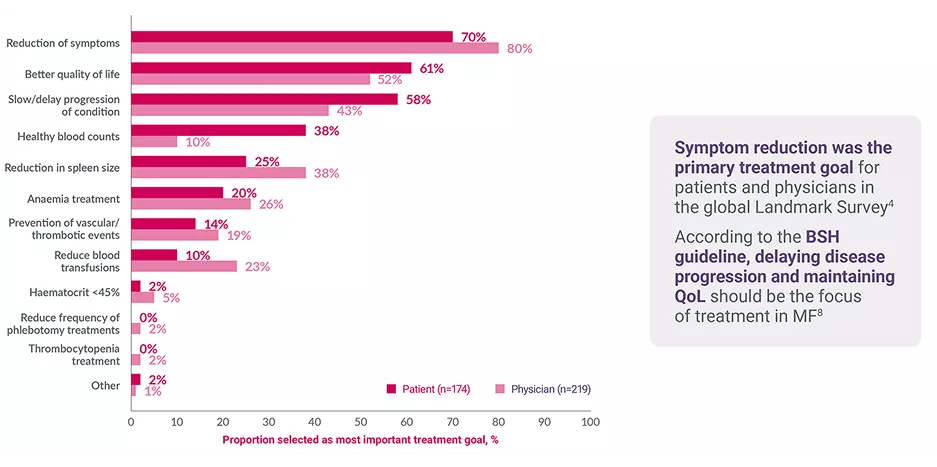
Patients with MF (n=174) regularly cancel plans, miss work and feel depressed or anxious4–7
The International MPN Landmark Survey was developed and funded by Novartis.
International MPN Landmark Survey: most important treatment goals in MF‡4
Adapted from Harrison CN, et al. 2017.4
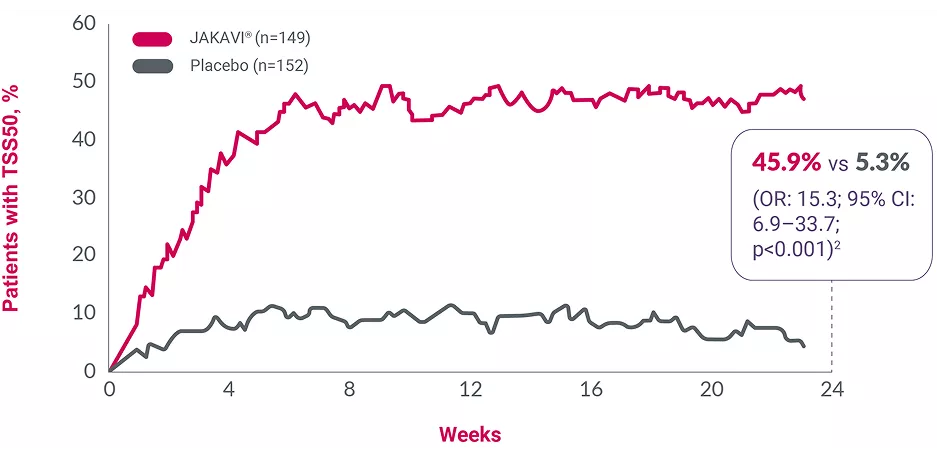
Adapted from Verstovsek S, et al. 2012.2
Assessed by the modified Myelofibrosis Symptom Assessment Form (MF-SAF) in a double-blind, Phase III RCT (intent-to-treat analysis)*§2
Negative scores indicate a decrease in symptoms; p-values were not reported.
The most frequently reported haematological adverse drug reactions included anaemia (83.8%), thrombocytopenia (80.5%) and neutropenia (20.8%). The three most frequent non-haematological adverse drug reactions were bruising (33.3%), other bleeding (including epistaxis, post-procedural haemorrhage and haematuria) (24.3%) and dizziness (21.9%).1 Please refer to the SmPC for more information.
*Data from patients with MF (n=174, including 45 UK patients) in the international MPN Landmark Survey (N=699, including 286 UK patients).4
†MF patients in full- or part-time employment at the time of the survey.4
‡Patients and physicians were asked to assign rankings for their top three treatment goals. Patients were asked: Other than a cure for your condition, what are your three most important treatment goals? Physicians were asked: Other than a cure, what is your most important treatment goal for therapy?4
§Improvements in symptoms were observed in patients treated with JAKAVI® at Week 4, maintained to Week 24; the primary endpoint was the proportion of patients with an SVR35 at Week 24. Each value plotted represents the moving average for the previous 7 days. Shortened 7-item modified MF-SAF form used, covering night sweats, itching, abdominal discomfort, pain under the ribs on the left side, early satiety, muscle/bone pain and inactivity.2
BAT, best available therapy; BSH, British Society for Haemtaology; CI, confidence interval; EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; int-2, intermediate-2; MF, myelofibrosis; MF-SAF, myelofibrosis symptom assessment form; MPN, myeloproliferative neoplasm; OR, odds ratio; PV, polycythaemia vera; QoL, quality of life; RCT, randomised controlled trial; SVR35, ≥35% spleen volume reduction; TSS, total symptom score.
References
JAKAVI® (ruxolitinib) Summary of Product Characteristics.
Verstovsek S, et al. N Engl J Med 2012;366:799–807 and supplementary appendix.
Harrison C, et al. N Engl J Med 2012;366:787–798.
Harrison CN, et al. Ann Hematol 2017;96:1653–1665.
Petruk C and Mathias J. Adv Ther 2020;37:2050–2070.
Brochmann N, et al. Clin Epidemiol 2019;11:23–33.
Yu J, et al. BMC Cancer 2018;18:420–424.
McLornan DP, et al. Br H Haematol 2024;204:136–150.
Mesa R, et al. J Clin Oncol 2017;35:3844–3850.
UK | September 2025 | FA-11448876
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.