Resources
Explore our range of resources which have been designed to support you in your management of patients.
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
JAKAVI® (ruxolitinib) is indicated for the treatment of disease-related splenomegaly or symptoms in adult patients with primary myelofibrosis (also known as chronic idiopathic myelofibrosis), post polycythaemia vera myelofibrosis or post essential thrombocythaemia myelofibrosis. JAKAVI® is also indicated for adult patients with polycythaemia vera who are resistant to or intolerant of hydroxyurea.1
For full safety information, please refer to the Summary of Product Characteristics.
Study | Type of study | Regimen | Study purpose and description | Study population |
|---|---|---|---|---|
COMFORT‑I2 | Phase III, double-blind, randomised controlled trial (RCT) | JAKAVI® (n=155) vs placebo (n=154) | Assessment of the efficacy and safety profile of JAKAVI® | Patients with intermediate-2 (int-2) or high-risk myelofibrosis (MF) |
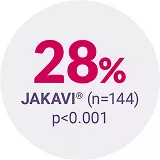
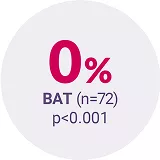
COMFORT‑II3 | Phase III, open-label RCT | JAKAVI® (n=146) vs BAT (n=73) | Assessment of the efficacy and safety profile of JAKAVI® | Patients with int-2 or high-risk MF, post-polycythaemia vera (PV) MF or post-essential thrombocythaemia (ET) MF |
Adapted from Harrison C, et al. 2012.3
In the COMFORT-I Phase III RCT, 41.9% and 0.7% of patients treated with JAKAVI® (n=155) vs placebo (n=154) achieved SVR35 at Week 24, respectively (primary endpoint, p<0.001).2
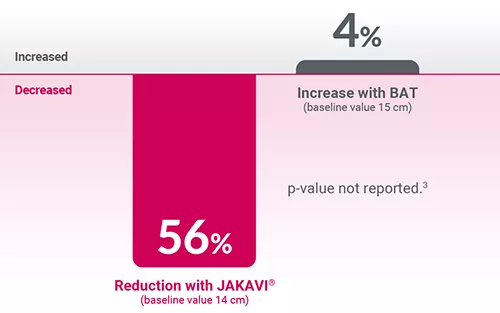
Adapted from Harrison C, et al. 2012.3
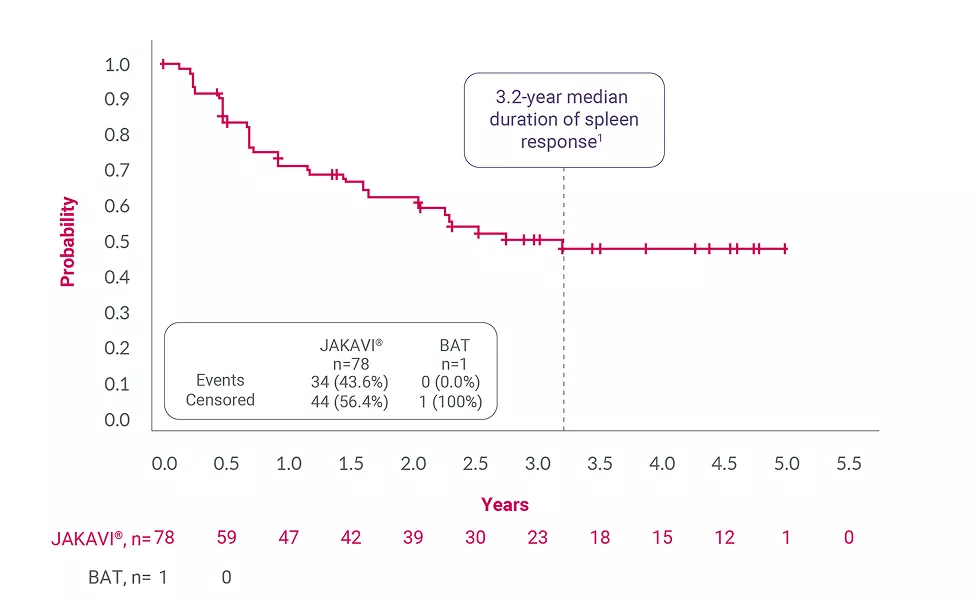
Adapted from Harrison C, et al. 2016.4
The most frequently reported haematological adverse drug reactions included anaemia (83.8%), thrombocytopenia (80.5%) and neutropenia (20.8%). The three most frequent non-haematological adverse drug reactions were bruising (33.3%), other bleeding (including epistaxis, post-procedural haemorrhage and haematuria) (24.3%) and dizziness (21.9%).1 Please refer to the Summary of Product Characteristics for more information.
*Patients treated with JAKAVI® achieved reduction in spleen length at Week 4 from baseline, confirmed by reduction in spleen volume at Week 24, which was maintained to Week 48. The median time to 1st CT/MRI observation of an SVR35 was 12.3 weeks.3
†Spleen response was defined as the interval from first spleen volume measurement of ≥35% reduction from baseline at any time on study and the first scan that is no longer a 35% reduction and that is a >25% increase over on-study nadir.4
BAT, best available therapy; CI, confidence interval; ET, essential thrombocythaemia; int-2, intermediate-2; MF, myelofibrosis; PV, polycythaemia vera; RCT, randomised controlled trial; SVR35, ≥35% spleen volume reduction.
References
JAKAVI® (ruxolitinib) Summary of Product Characteristics.
Verstovsek S, et al. N Engl J Med 2012;366:799–807.
Harrison C, et al. N Engl J Med 2012;366:787–798 and supplementary appendix.
Harrison C, et al. Leukemia 2016;30:1701–1707.
UK | September 2025 | FA-11448865-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.