
ENTRESTO® (sakubitril/valsartan)
Indikacije zdravila ENTRESTO® (sakubitril/valsartan):
Srčno popuščanje pri odraslih: zdravljenje simptomatskega kroničnega srčnega popuščanja z zmanjšanim iztisnim deležem pri odraslih bolnikih.
Srčno popuščanje pri pediatričnih bolnikih: zdravljenje simptomatskega kroničnega srčnega popuščanja s sistolično disfunkcijo levega prekata pri otrocih in mladostnikih, ki so stari eno leto ali več.1
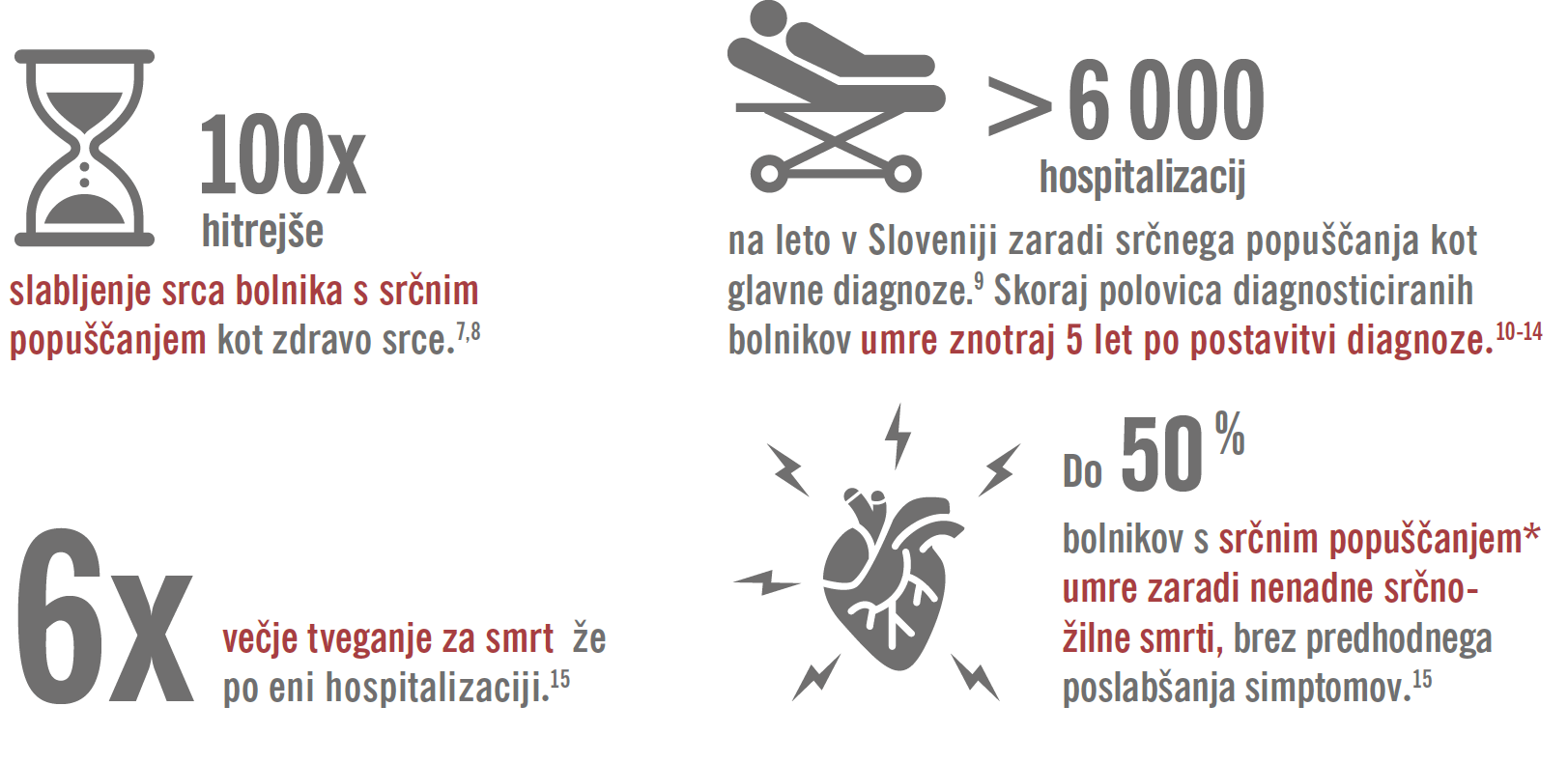
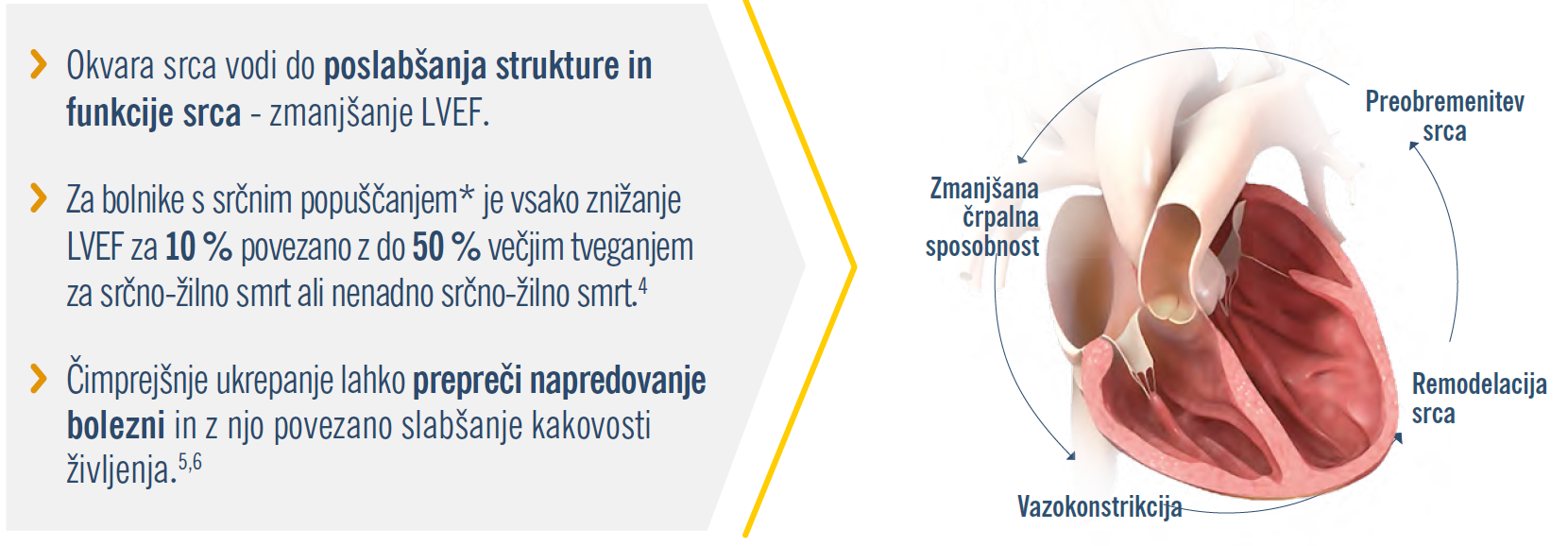
Okvare srca s časom napredujejo, čeprav tega ne vidimo.4
Naredite spremembo, ki jo vaš bolnik lahko čuti.1-3
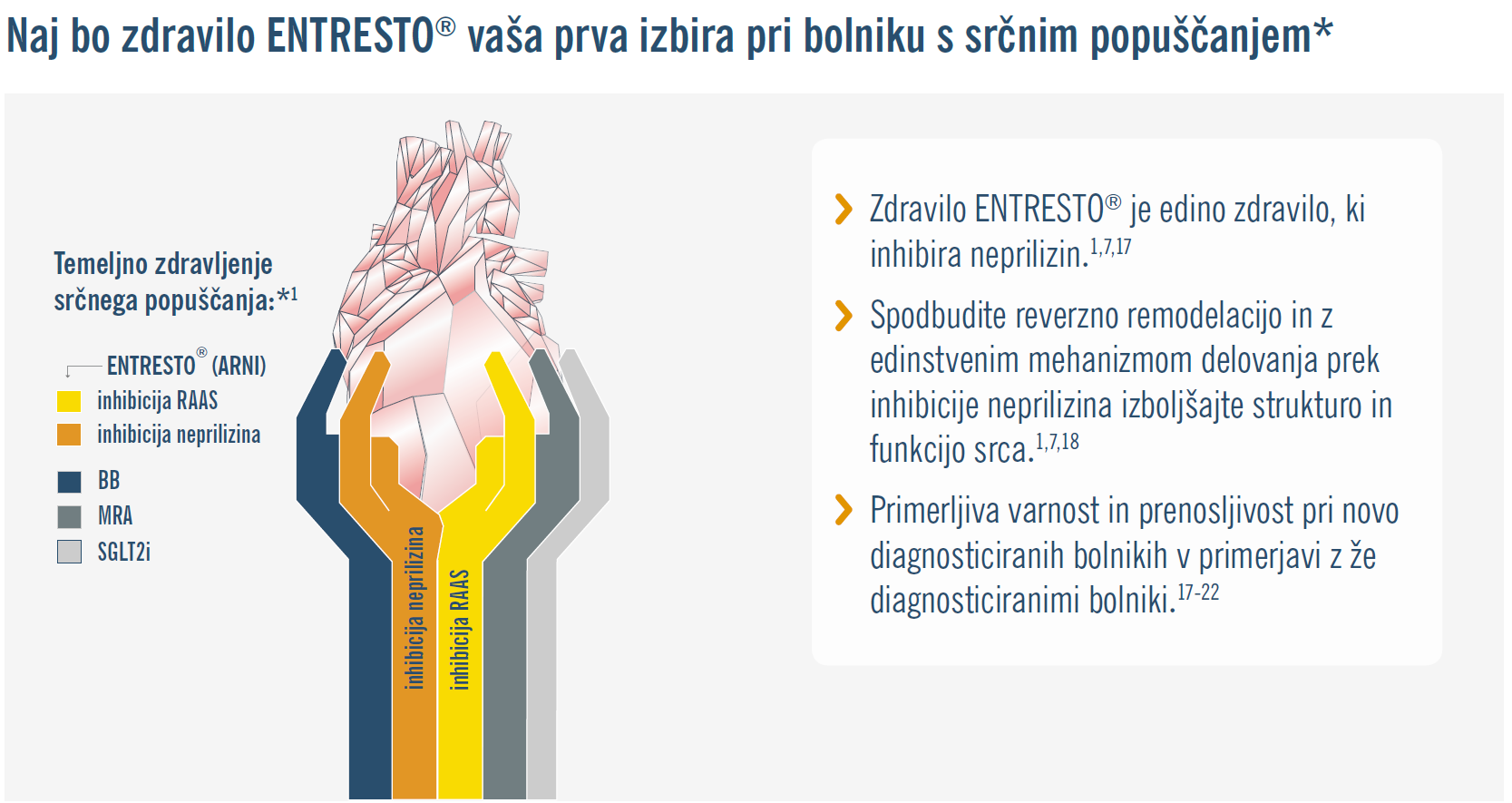
* z zmanjšanim iztisnim deležem levega prekata.
Legenda:
ACE=angiotenzinska konvertaza (angl.: angiotensin converting enzyme); ARB =zaviralec angiotenzinskih receptorjev (angl.: angiotensin receptor blocker); ARNI=zaviralec angiotenzinskih receptorjev in neprilizina - edini predstavnik je sakubitril/valsartan (angl.: angiotensin receptor–neprilysin inhibitor); BB=zaviralec beta (angl.: beta blocker); LVEF=iztisni delež levega prekata (angl. left ventricular ejection fraction); MRA=zaviralec mineralokortikoidnih receptorjev (angl.: mineralocorticoid receptor antagonist); SGLT2i=zaviralec natrij-glukoznega prenašalca 2 (angl.: sodium-glucose cotransporter-2 inhibitor).
Reference:
Povzetek glavnih značilnosti zdravila Entresto 24 mg/26 mg, 49 mg/51 mg in 97 mg/103 mg filmsko obložene tablete. Datum zadnje revizije besedila maj 2025.
Claggett B, Packer M, McMurray JJV, et al; for the PARADIGM-HF Investigators. Estimating the long-term treatment benefits of sacubitril–valsartan. N Engl J Med. 2015;373(23):2289-2290.
Lewis EF, Claggett BL, McMurray JJV, et al. Health-related quality of life outcomes in PARADIGM-HF. Circ Heart Fail. 2017;10(8):e003430.
Solomon SD, Anavekar N, Skali H, et al; for the Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators. Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation. 2005;112(24):3738–3744.
Maddox TM, Januzzi JL Jr, Allen LA, et al. 2021 update to the 2017 ACC Expert Consensus Decision Pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810.
Vaduganathan M, Claggett BL, Jhund PS, et al. Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020;396(10244):121–128.
Menendez JT. The mechanism of action of LCZ696. Card Fail Rev. 2016;2(1):40-46.
Konstantinidis K, Whelan RS, Kitsis RN. Mechanisms of cell death in heart disease. Arterioscler Thromb Vasc Biol. 2012;32(7):1552-1562.
Omersa et al. National trends in heart failure hospitalization rates in Slovenia 2004–2012. European Journal of Heart Failure (2016) 18, 1321–1328.
Roger et al. JAMA 2004;292:344–50;
Levy et al. N Engl J Med 2002;347:1397–402;
Go et al. Circulation 2014;129:e28-e292;
Yancy et al. Circulation 2013;128:e240–327;
Ahmed. Am J Cardiol 2007;99:549–53
Okumura N, Jhund PS, Gong J, et al; for the PARADIGM-HF Investigators and Committees. Importance of clinical worsening of heart failure treated in the outpatient setting: evidence from the Prospective comparison of ARNI with ACEi to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Circulation. 2016;133(23):2254–2262.
Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41(18):1757–1763.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;00:1–128.
Januzzi JL Jr, Prescott MF, Butler J, et al; for the PROVE-HF Investigators. Association of change in N-terminal pro–b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322(11):1085-1095.
Heidenreich P, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145(18):e895–e1032.
Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail.
2019;21(10):1169-1186.Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/ valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139(19):2285–2288.
Senni M, Wachter R, Witte KK, et al. Initiation of sacubitril/valsartan shortly after hospitalization for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2019;1-doi:10.1002/ejhf.1670.
FA-11513270

