
ENTRESTO® (sakubitril/valsartan)
Indikacije zdravila ENTRESTO® (sakubitril/valsartan):
Srčno popuščanje pri odraslih: zdravljenje simptomatskega kroničnega srčnega popuščanja z zmanjšanim iztisnim deležem pri odraslih bolnikih.
Srčno popuščanje pri pediatričnih bolnikih: zdravljenje simptomatskega kroničnega srčnega popuščanja s sistolično disfunkcijo levega prekata pri otrocih in mladostnikih, ki so stari eno leto ali več.1
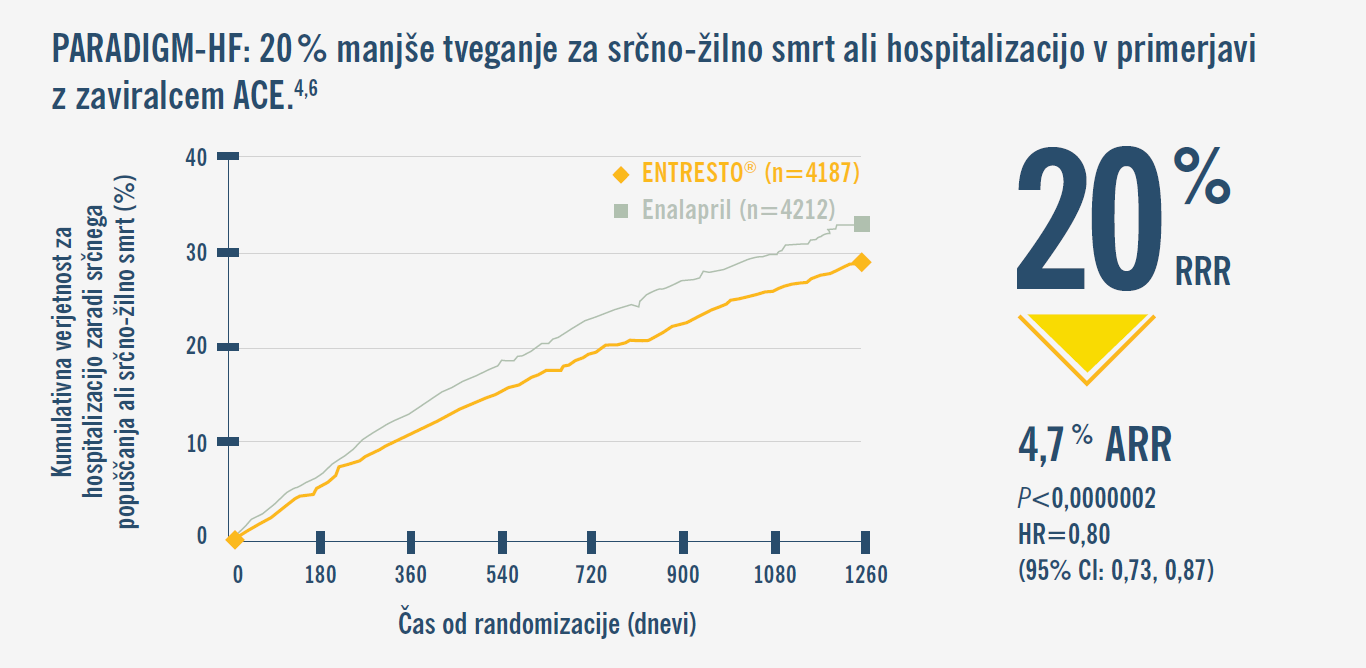
Odločite se za zdravilo ENTRESTO® in zaščitite bolnika kjerkoli na njegovi poti.1,2,10,14
Začnite terapijo že v bolnišnici ali ambulanti, tako pri naivnem† ali novo diagnosticiranem bolniku kot pri simptomatskem bolniku na terapiji.
† Bolnik, ki še ni bil zdravljen z zaviralcem ACE ali ARB.
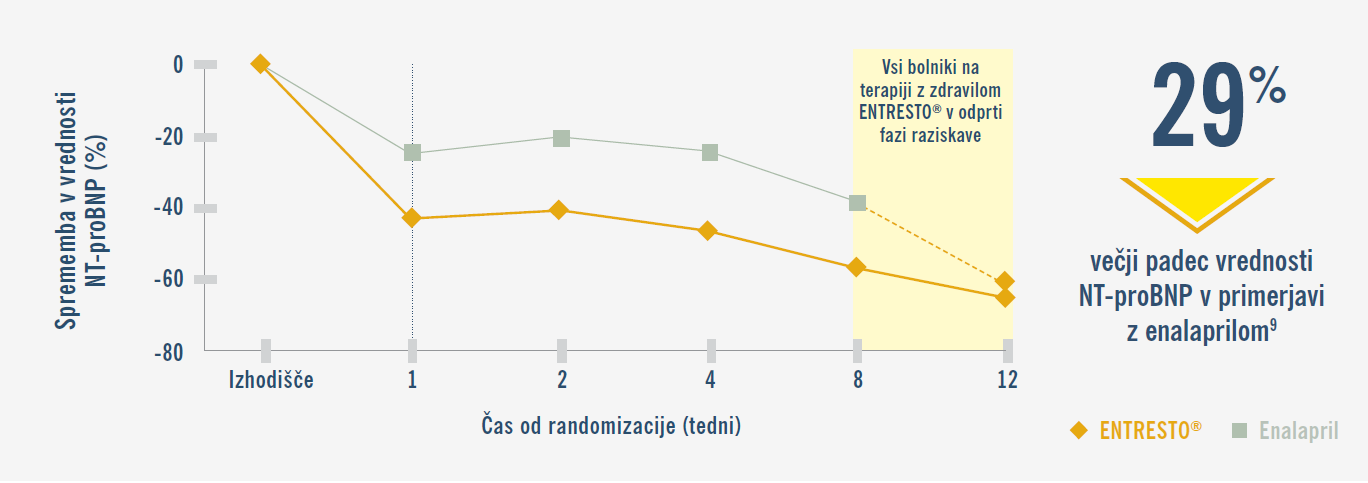
Primerljivo zmanjšanje NT-proBNP vrednosti.2,3
Primerljivo izboljšanje parametrov reverzne remodelacije srca pri novo diagnosticiranih bolnikih v primerjavi z že diagnosticiranimi bolniki.2
Primerljiva varnost in prenosljivost pri novo diagnosticiranih bolnikih v primerjavi z že diagnosticiranimi bolniki.4,5
Novo diagnosticiran bolnik
Za novo diagnosticiranega bolnika s srčnim popuščanjem* izberite zdravilo ENTRESTO® in izboljšajte potek bolezni.2
Delež novo diagnosticiranih bolnikov v različnih raziskavah:2,3,6
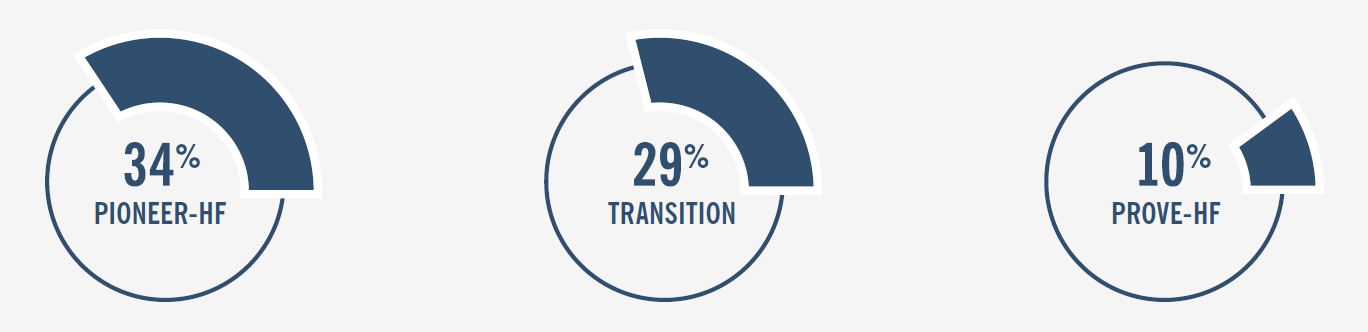
PIONEER-HF: Kumulativna pojavnost ponovne hospitalizacije ali srčno-žilne smrti.7
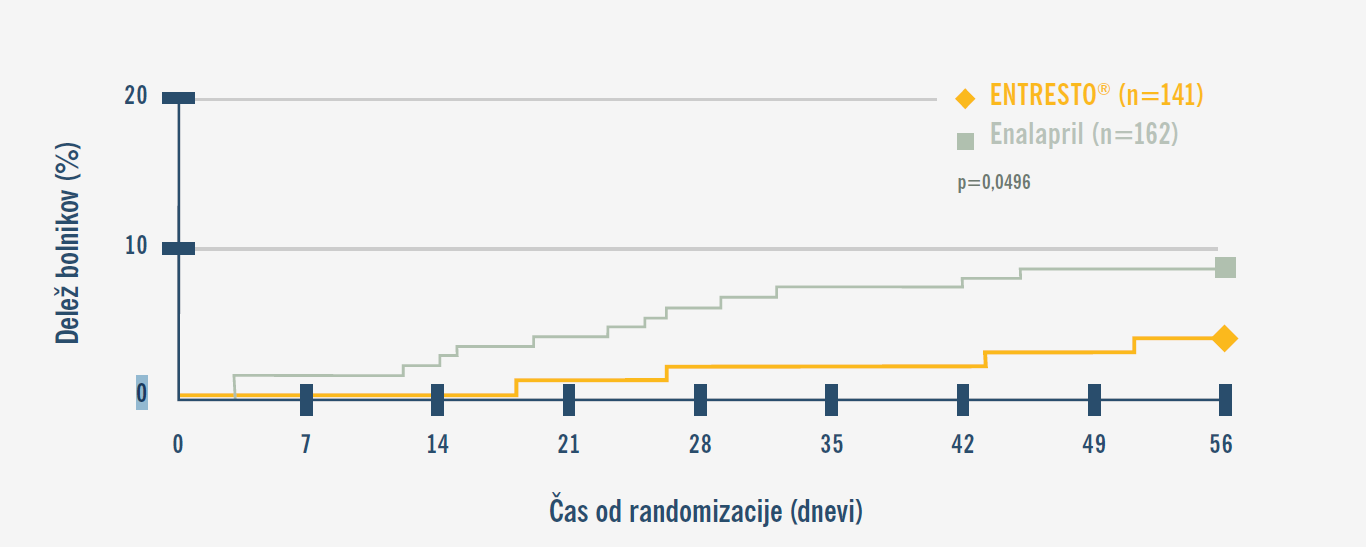
Uvedba zdravila ENTRESTO® pri novo diagnosticiranih bolnikih zmanjša tveganje za ponovno hospitalizacijo in srčno-žilno smrt v primerjavi z zaviralci ACE.7
Hospitaliziran bolnik
Ne odlašajte.8,9
Začnite z zdravilom ENTRESTO® in zaščitite srce bolnika že v bolnišnici.6,10-13
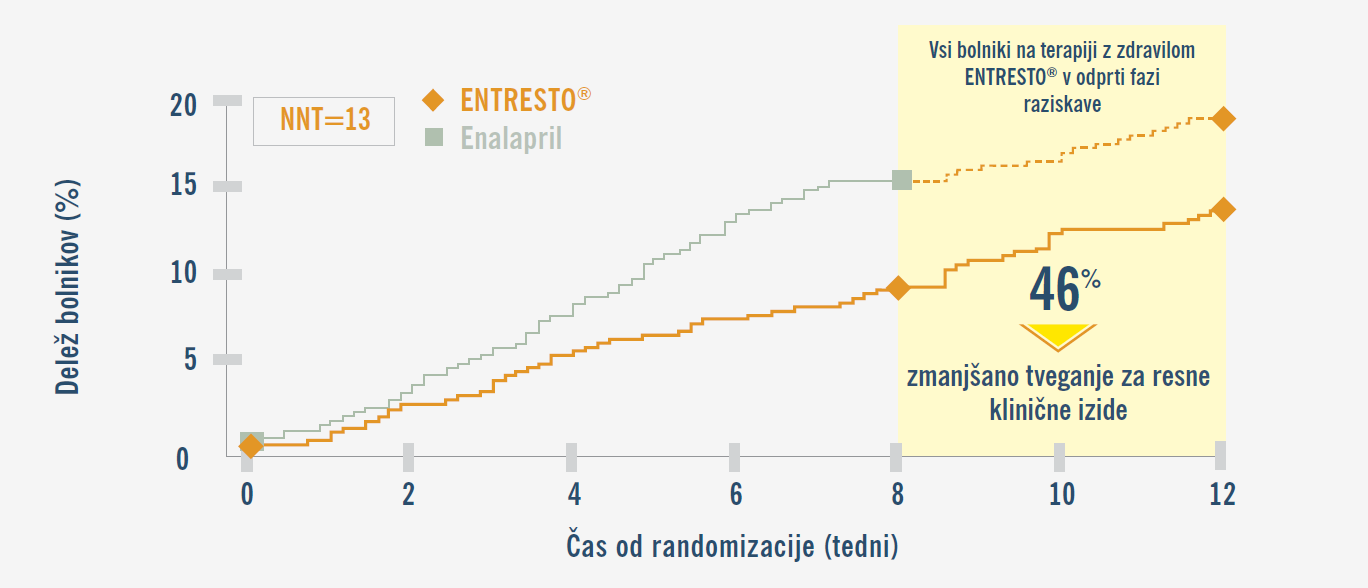
PIONEER-HF: Uvajanje zdravila ENTRESTO® v bolnišnici vodi do značilno večjega in hitrejšega zmanjšanja vrednosti NT-proBNP v primerjavi z enalaprilom.9,19
Ambulantni bolnik

* z zmanjšanim iztisnim deležem levega prekata.
§ Sprememba v terapiji - nov recept, menjava terapije in/ali dodatna terapija.
Začnite z zdravilom ENTRESTO® in pomagajte bolniku ostati izven bolnišnice in živeti dlje.1,15,16

Legenda:
ACE = angiotenzinska konvertaza (angl.: angiotensin converting enzyme); ARB = zaviralec angiotenzinskih receptorjev (angl.: angiotensin receptor blocker); ARR=zmanjšanje absolutnega tveganja (angl.: absolute risk reduction); HR=razmerje ogroženosti (angl.: hazard ratio); IZ=interval zaupanja (angl.: confidence interval); RRR=zmanjšanje relativnega tveganja (angl.:relative risk reduction); NNT=število bolnikov, ki jih je treba zdraviti za preprečitev neželenega izida (angl.: number needed to treat); LVAD=terapija z mehansko podporo levega prekata (angl.: left ventricle assist device).
Reference:
Povzetek glavnih značilnosti zdravila Entresto 24 mg/26 mg, 49 mg/51 mg in 97 mg/103 mg filmsko obložene tablete. Datum zadnje revizije besedila maj 2025.
Senni M, Wachter R, Witte KK, et al. Initiation of sacubitril/valsartan shortly after hospitalization for acutely decompensated heart failure in patients with newly diagnosed (de novo) heart failure: a subgroup analysis of the TRANSITION study. Eur J Heart Fail. 2019;1-11. doi:10.1002/ejhf.1670.
Januzzi JL Jr, Prescott MF, Butler J, et al; for the PROVE-HF Investigators. Association of change in N-terminal pro–b-type natriuretic peptide following initiation of sacubitril-valsartan treatment with cardiac structure and function in patients with heart failure with reduced ejection fraction. JAMA. 2019;322(11):1085-1095.
Seferovic PM, Ponikowski P, Anker SD, et al. Clinical practice update on heart failure 2019: pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2019;21(10):1169-1186.
Hollenberg SM, Stevenson LW, Ahmad T, et al. 2019 ACC expert consensus decision pathway on risk assessment, management, and clinical trajectory of patients hospitalized with heart failure: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2019;74(15):1966-2011.
Velazquez EJ, Morrow DA, DeVore AD, et al; for the PIONEER-HF Investigators. Angiotensin–neprilysin inhibition in acute decompensated heart failure. N Engl J Med. 2019;380(6):539-548.
Ambrosy AP, Braunwald E, Morrow DA, et al. Angiotensin receptor-neprilysin inhibition based on history of heart failure and use of renin-angiotensin system antagonists. J Am Coll Cardiol. 2020;76(9):1034–1048.
Maddox TM, Januzzi JL Jr, Allen LA, et al. 2021 update to the 2017 ACC Expert Consensus Decision Pathway for optimization of heart failure treatment: answers to 10 pivotal issues about heart failure with reduced ejection fraction: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772–810.
McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2021;00:1–128.
Morrow DA, Velazquez EJ, DeVore AD, et al. Clinical outcomes in patients with acute decompensated heart failure randomly assigned to sacubitril/valsartan or enalapril in the PIONEER-HF trial. Circulation. 2019;139(19):2285–2288.
DeVore AD, Braunwald E, Morrow AD, et al; for the PIONEER-HF Investigators. Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open-label extension of the PIONEER-HF trial. JAMA Cardiol. 2020;5(2):202–207.
DeVore AD, Braunwald E, Morrow AD, et al; for the PIONEER-HF Investigators. Initiation of angiotensin-neprilysin inhibition after acute decompensated heart failure: secondary analysis of the open-label extension of the PIONEER-HF trial. JAMA Cardiol. 2020;5(2) (suppl):202–207. Accessed January 2022. Supplementary material accessed at https://jamanetwork.com/journals/jamacardiology/article-abstract/2756356(link is external)
Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ. 1995;310:452–454.
Proudfoot C, Studer R, Rajput T, et al. Real-world effectiveness and safety of sacubitril/valsartan in heart failure: A systematic review. Int J Cardiol. 2021;15(331):164–171.
Packer M. What causes sudden death in patients with chronic heart failure and a reduced ejection fraction? Eur Heart J. 2020;41(18):1757–1763.
Al-Gobari M, Al-Aqeel S, Gueyffier F, et al. Effectiveness of drug interventions to prevent sudden cardiac death in patients with heart failure and reduced ejection fraction: an overview of systematic reviews. BMJ Open. 2018;8:e021108.
Data on file. IMS CHF Indication Xponent 3-month data ending Oct 2018. Novartis Pharmaceuticals Corp; 2018.
FA-11513270

