

TAFINLAR® (dabrafenib) + MEKINIST® (trametinib) dosing and administration
TAFINLAR in combination with MEKINIST is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
Helping patients on their TAFINLAR + MEKINIST treatment journey
The management of adverse events (AEs) may require treatment interruption, dose reduction, or treatment discontinuation. For information on AE management with dose modification, and dosing in special populations, please click here and refer to the SmPC.
TAFINLAR + MEKINIST is an oral option in the adjuvant setting1,2
Dosing is the same for both adjuvant and metastatic patients with melanoma1,2
In the metastatic setting, treatment with both TAFINLAR and MEKINIST should continue until the patient no longer derives benefit or develops unacceptable toxicity1,2
In the adjuvant setting, patients should be treated for a period of 12 months unless there is disease recurrence or unacceptable toxicity1,2
This key information on the dosing and administration of TAFINLAR + MEKINIST may be helpful when discussing treatment with your patients.
Please also visit the section on AE management, which includes details on how to manage dose modifications of TAFINLAR + MEKINIST should this be necessary, as well as information on dosing in special populations.
TAFINLAR + MEKINIST offers twice daily oral dosing1,2
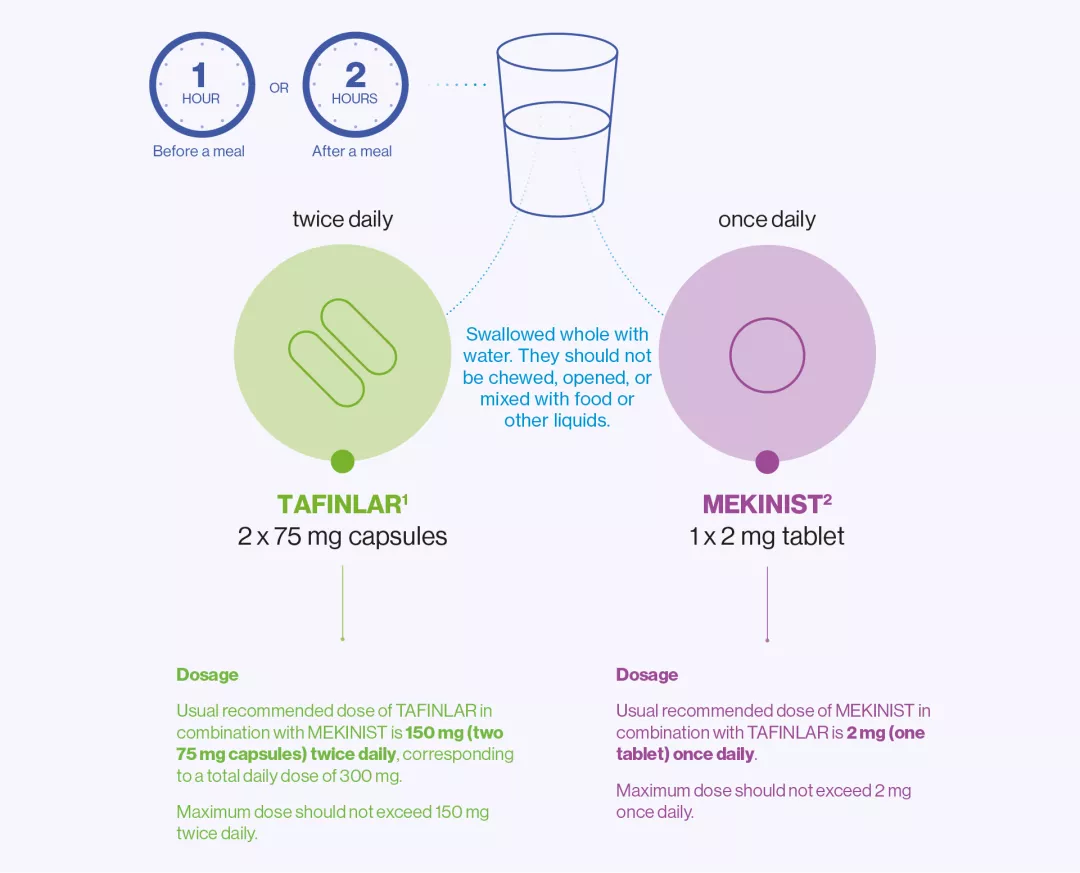
Dosing of TAFINLAR + MEKINIST
Drug | TAFINLAR1 | MEKINIST2 |
Usual recommended dose | Dose of TAFINLAR in combination with MEKINIST is 150 mg (two 75 mg capsules) twice daily, corresponding to a total daily dose of 300 mg | Dose of MEKINIST in combination with TAFINLAR is 2 mg (one tablet) once daily |
Maximum dose | The dose should not exceed 150 mg twice daily | The dose should not exceed 2 mg once daily |
Duration of treatment
In the metastatic treatment of melanoma, patients should be treated with TAFINLAR + MEKINIST until the patient no longer derives benefit or develops unacceptable toxicity.1,2
In the adjuvant treatment of melanoma, patients should be treated for a period of 12 months unless there is disease recurrence or unacceptable toxicity.1,2
The management of AEs associated with TAFINLAR + MEKINIST treatment may require dose reduction, treatment interruption or treatment discontinuation.1,2
For more information on dose modification guidelines please refer to the TAFINLAR + MEKINIST SmPCs and visit the dose modification tab where this is described in detail.
Administration of TAFINLAR + MEKINIST
How to take TAFINLAR + MEKINIST1,2
| TAFINLAR1 | MEKINIST2 |
How much to take | The usual dose is 150 mg (two 75 mg capsules) twice daily. | The usual dose is 2 mg (one tablet) once a day. |
When to take | TAFINLAR should be taken twice a day, in the morning and in the evening, about 12 hours apart. The morning and evening doses of TAFINLAR should be taken at similar times every day. | MEKINIST should be taken once a day, at about the same time each day, either with the morning or with the evening dose of TAFINLAR. |
How to take | TAFINLAR capsules should be swallowed whole with water. They should not be chewed or opened and should not be mixed with food or liquids, due to chemical instability of TAFINLAR. TAFINLAR should be taken on an empty | MEKINIST should be taken orally with a full glass of water. The tablets should not be chewed or crushed and they should be taken on an empty stomach, at least 1 hour before or 2 hours after a meal. |
Figures for illustrative purposes only
Practical advice for patients on how to take their treatment and how to incorporate it into their daily lives can be found here. Please do not share this page directly with patients.
| TAFINLAR | MEKINIST |
If a patient forgets to take TAFINLAR or MEKINIST | If the missed dose is less than 6 hours late, the patient must take it as soon as they remember. If the missed dose is more than 6 hours late, the patient should skip that dose and take the next dose at the usual time. Then carry on taking the capsules at regular times as usual. The patient must not take a double dose to make up for a missed dose. | If the missed dose is less than 12 hours late, the patient must take it as soon as they remember. If the missed dose is more than 12 hours late, the patient should skip that dose and take the next dose at the usual time.Then carry on taking the capsules at regular times as usual. The patient must not take a double dose to make up for a missed dose. |
If a patient vomits after taking TAFINLAR or MEKINIST | The patient must not take the capsules or tablet again but wait until next dose is due and take it at the normal time. | |
If a patient has taken more than the prescribed number of TAFINLAR capsules, MEKINIST tablets, or other medicines | The patient must consult a doctor, pharmacist or nurse immediately, and take the medicine(s) and its packaging with them if seeing them face to face. | |
Handling and storage of TAFINLAR and MEKINIST
Handling of TAFINLAR and MEKINIST
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.1,2
Storage of TAFINLAR and MEKINIST
For storage of TAFINLAR and MEKINIST, please refer to SmPC.
Additional information is also available from your Novartis representative, who can provide you with a comprehensive healthcare professional (HCP) brochure about TAFINLAR + MEKINIST.
BRAF V600, mutation of the BRAF gene at valine (V) 600; HCP, healthcare professional; PIL, patient information leaflet; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
UK | March 2025 | FA-11220691
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

