Pooled COMBI-v and COMBI-d efficacy
TAFINLAR® (dabrafenib) in combination with MEKINIST® (trametinib) is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
Common adverse events include:
TAFINLAR + MEKINIST: The most common adverse reactions (incidence ≥20%) for dabrafenib in combination with trametinib were pyrexia, fatigue, nausea, chills, headache, diarrhoea, vomiting, arthralgia and rash1,2
TAFINLAR: The most common adverse reactions (incidence >15%) reported with dabrafenib were hyperkeratosis, headache, pyrexia, arthralgia, fatigue, nausea, papilloma, alopecia, rash and vomiting1
MEKINIST: The most common adverse reactions (incidence ≥20%) for trametinib were rash, diarrhoea, fatigue, oedema peripheral, nausea and dermatitis acneiform2
For more safety information on TAFINLAR and MEKINIST, click here.
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
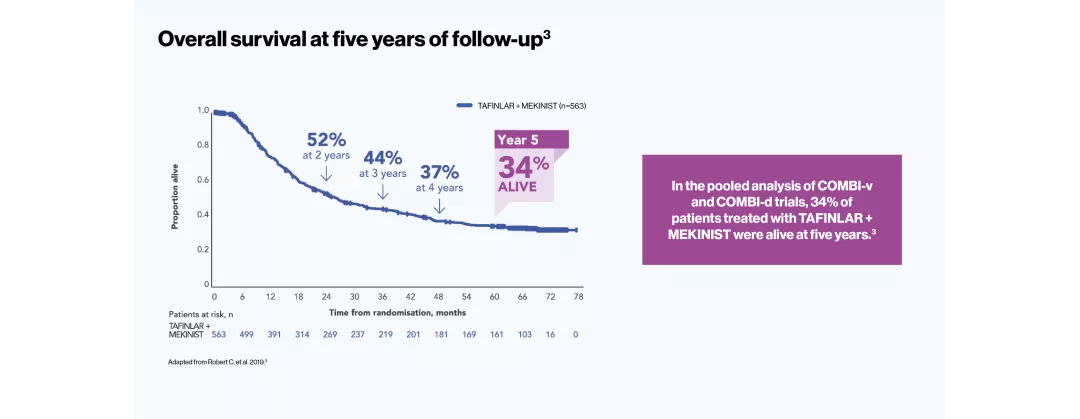
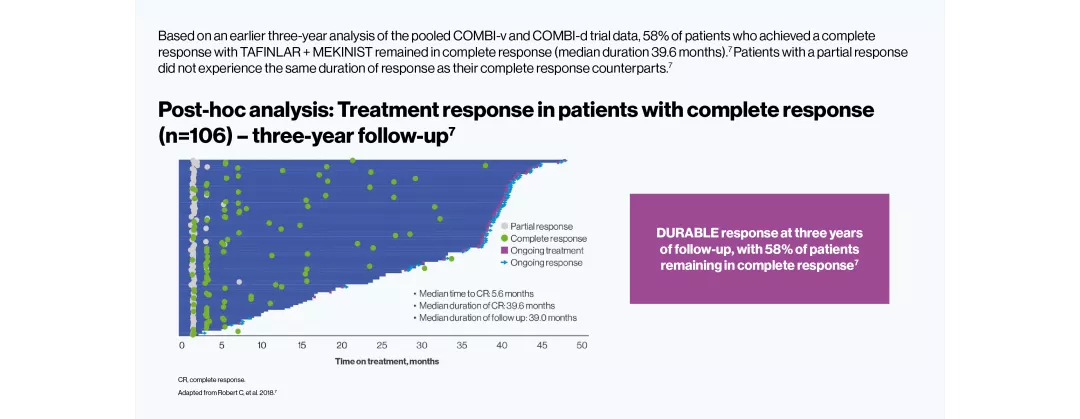
DURABLE five-year survival responses3
TAFINLAR + MEKINIST showed DURABLE responses up to five years in adult patients with BRAF V600-positive unresectable or metastatic melanoma3
Key clinical data insights from COMBI-v and COMBI-d
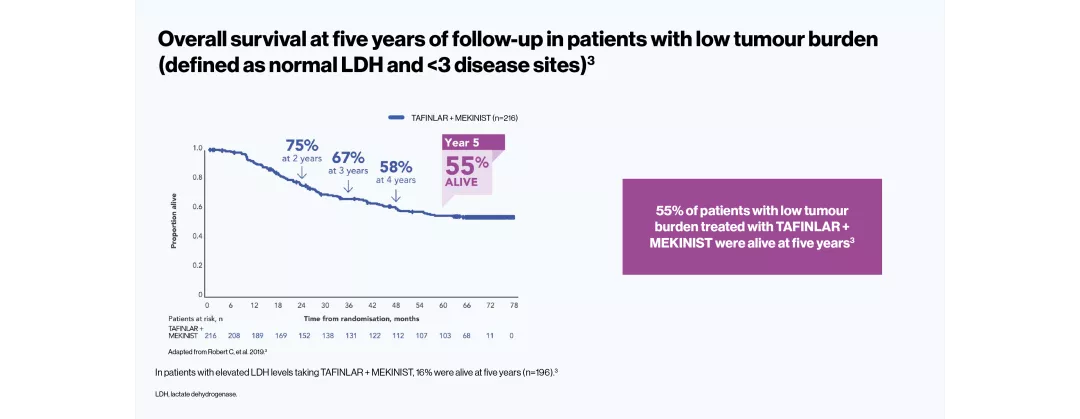
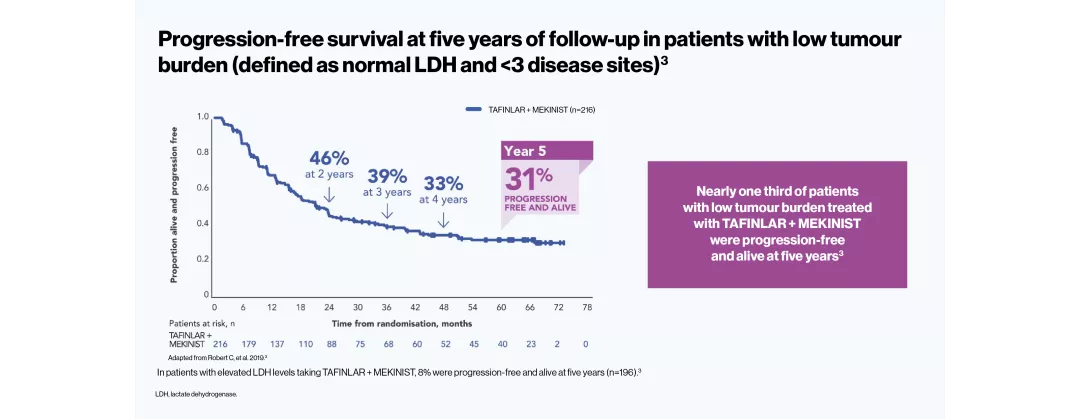
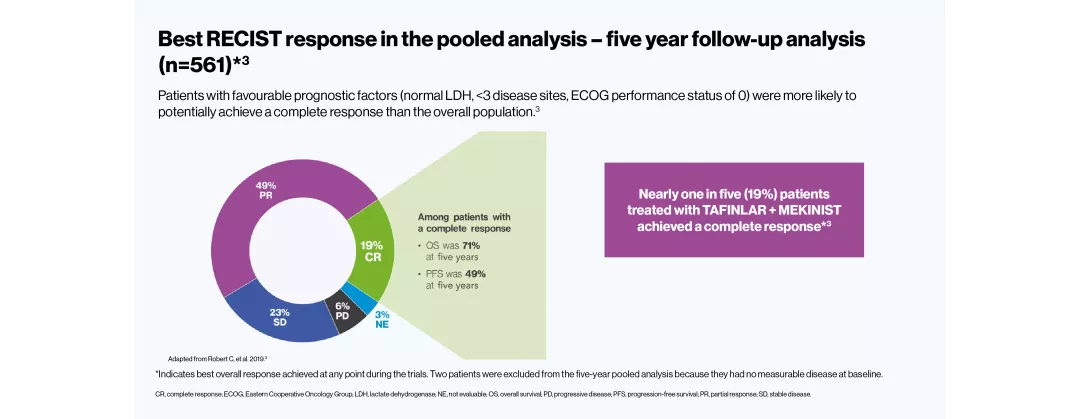
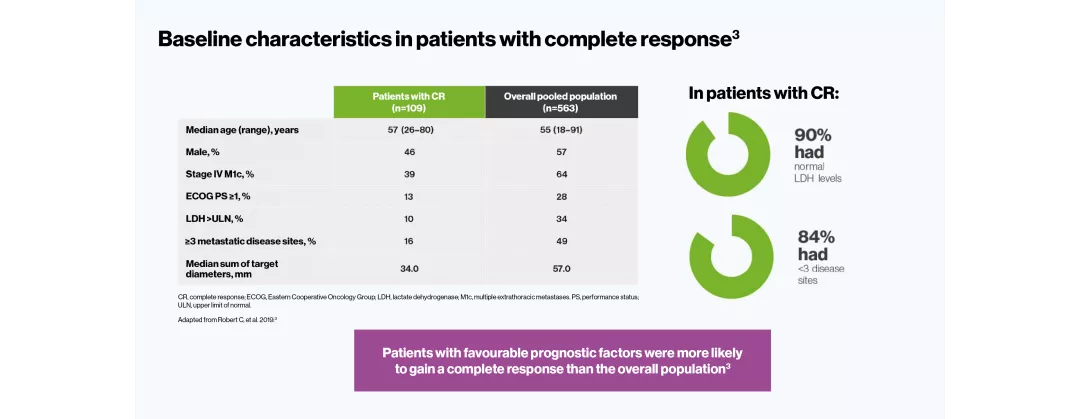
The efficacy and safety profile of TAFINLAR + MEKINIST for the treatment of adult patients with BRAF V600-positive unresectable or metastatic melanoma has been studied in two large Phase III trials: COMBI-v (vs vemurafenib) and COMBI-d (vs dabrafenib).4–6 A pooled analysis (N=563) was performed on the data from the TAFINLAR + MEKINIST treatment arms of these two trials. In a landmark five-year analysis of the trials, TAFINLAR + MEKINIST demonstrated that:
Please visit the Pooled COMBI-v and COMBI-d safety profile page for further information on the TAFINLAR + MEKINIST safety profile in the adjuvant setting.
COMBI-v and COMBI-d study design
The pooled population consisted of 563 first-line adult patients with BRAF V600-positive unresectable or metastatic melanoma. All patients in the analysis had been randomised to receive TAFINLAR 150 mg BID + MEKINIST 2 mg QD in either COMBI-v or COMBI-d. Median follow-up was 22 months (range, 0–76).3

aCrossover was prohibited until the independent data and safety monitoring committee recommended stopping the study early due to superior efficacy in the combination arm. After the recommendation (July 2014), the study protocol was amended to allow patients in the vemurafenib group to cross over to the combination group.4
bCrossover was not allowed during the course of the study, in order to preserve the integrity of the OS endpoint. However, after the 2-year OS data cutoff, crossover was subsequently followed and may confound future OS analyses.5,6
cDefined as time from randomisation to death from any cause.4
dDefined as the time from randomisation until radiologic disease progression or death from any cause.5
*Indicates best overall response achieved at any point during the trials.
BID, twice daily; BRAF V600E/K, mutation of the BRAF gene at valine (V) 600 to either glutamate (E) or lysine (K); DOR, duration of response; ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; MEK, mitogen-activated extracellular signal-regulated kinase; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QD, once daily; R, randomised; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib). Summary of Product Characteristics.
MEKINIST (trametinib). Summary of Product Characteristics.
Robert C, et al. N Engl J Med 2019;381:626–636.
Robert C, et al. N Engl J Med 2015;372:30–39.
Long G, et al. N Engl J Med 2014;371:1877–1888.
Long G, et al. Lancet 2015;386:444–451(inc. suppl).
Robert C, et al. Pigment Cell Melanoma Res 2018;31:201.
UK | May 2025 | FA-11218759-2
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.