

Diagnosis and treatment of melanoma
TAFINLAR in combination with MEKINIST is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
Melanoma is the fifth most common cancer in the UK;3 however, earlier diagnosis can improve the prognosis4
Melanoma is a heterogenous disease that can be difficult to diagnose and treat.4 In its early stages, melanoma can be successfully treated with surgery alone.4 However, survival rates fall significantly once melanoma has metastasised. Early and accurate diagnosis is therefore crucial for giving patients their best chance of survival.4
Improvements in the understanding of the mutational causes of melanoma have led to the development of treatments, including targeted therapies and immunotherapies. Offering a more personalised approach to treatment may not only contribute to improved prognosis for patients with melanoma, but help with cost savings and improved sustainability of healthcare systems.4
Accurate staging of melanoma is crucial to guide treatment4
ABCDEs of melanoma5
A patient will usually consult their general practitioner (GP) if they have concerns about a suspect mole or skin lesion. The GP will use the simple visual ABCDE method to determine if the patient should be referred to a dermatologist or an oncologist for further tests.
Representation of a melanoma – ABCDE identification5
A. Asymmetry: the two halves of the area may differ in shape.
B. Border: the edges of the area may be irregular or blurred, and sometimes show notches.
C. Colour: this may be uneven. Different shades of black, brown and pink may be seen.
D. Diameter: change in size, usually an increase. Melanomas can be tiny, but most are larger than the size of a pea (>6 mm).
E. Evolution: change in size, shape, colour or other traits.
Staging melanoma6–8
Staging has an impact on prognostic assessment and treatment decision making.4 There are several different staging systems in current use. The system most commonly used is the American Joint Committee on Cancer (AJCC) TNM system, which is based on tumour size (T), lymph node involvement (N) and metastasis (M).6–8
Other staging systems used at biopsy include the Breslow Depth,9 that measures depth or thickness of the primary tumour, and the Clark Level,8 that examines the anatomical depth into subcutaneous tissue. These systems are not interchangeable, but offer useful measures when staging melanoma.7 These three systems are described below.
AJCC staging uses three pieces of information7,8
Tumour size (T) | Lymph node involvement (N) | Metastasis (M) |
The thickness of the primary tumour, and if it is ulcerated | The number of lymph nodes involved, and if they are regional or nearby | The degree of disease spread throughout the body |
Tis, melanoma in situ T1, ≤1.0 mm T2, >1.0–2.0 mm T3, >2.0–4.0 mm T4, >4.0 mm | N0, no regional metastases N1, one tumour-involved node or in-transit, satellite, and/or microsatellite metastases N2, two or three tumour-involved nodes or in-transit, satellite, and/or microsatellite metastases with one tumour-involved node N3, four or more tumour-involved nodes or in-transit, satellite, and/or microsatellite metastases with two or more tumour-involved nodes, or any number of matted nodes without or with in-transit satellite, and/or microsatellite metastases | M0, no evidence of distant metastasis M1, evidence of distant metastasis |
TNM pathological staging overview7,8
Stage | Tumour | Lymph node involvement | Metastasis |
0 | Tis | N0 | M0 |
IA | T1a or T1b | N0 | M0 |
IB | T2a | N0 | M0 |
IIA | T2b or T3a | N0 | M0 |
IIB | T3b or T4a | N0 | M0 |
IIC | T4b | N0 | M0 |
IIIA | T1a/b or T2a | N1a or N2a | M0 |
IIIB | T0 T1a/b or T2a T2b or T3a | N1b or N1c N1b/cor N2b N1a/b/c or N2a/b | M0 |
IIIC | T0 T1a/b or T2a/b, or T3a T3b or T4a T4b | N2b/c or N3b/c N2c or N3a/b/c Any N≥N1 N1a/b/c or N2a/b/c | M0 |
IIID | T4b | N3a/b/c | M0 |
IV | Any T, Tis | Any N | M1 |
Breslow classification system9
Breslow Depth details the prognostic significance of the thickness of a melanoma. It is based on the depth of invasion in millimeters (mm) rather than the depth by anatomic compartments. Patients with thinner melanomas (Stages I and II) have a much better chance of survival and a lower risk of regional and distant metastasis than those with thicker melanomas (Stages III to V).7
Tumours are classified into four categories based on depth:7
Less than or equal to 0.75 mm
0.76–1.5 mm
1.51–4 mm
Greater than or equal to 4 mm
Clark Level10
The Clark method looks at the invasion of melanoma cells into the dermis and subcutaneous fat, and the risk of spread to lymph nodes and distant organs.6–8 There are five levels in the Clark staging system.
AJCC staging uses three pieces of information7,8
Stage | Melanoma stage description | Prognosis (as of 2018)*10 |
Image
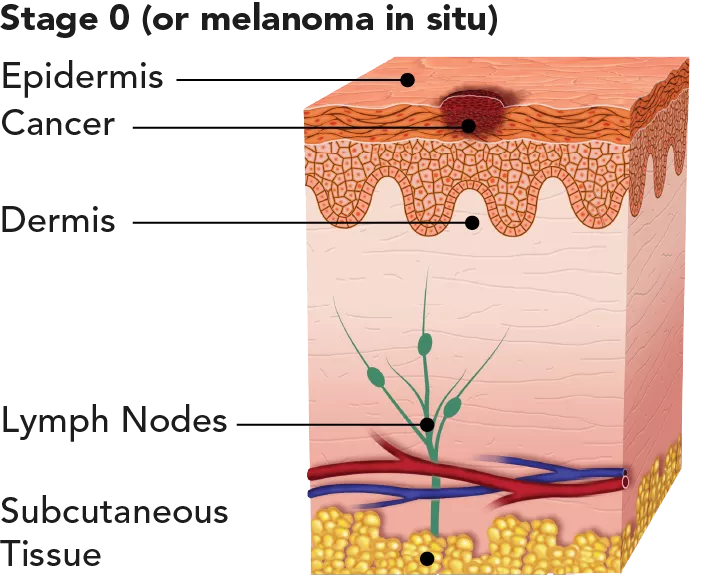
| The tumour is only in the epidermis, the outer layer of the skin, and hasn’t spread deeper. | Highly curable, with very little risk for recurrence or metastasis. 5-year survival rate: 98.4%. |
Image
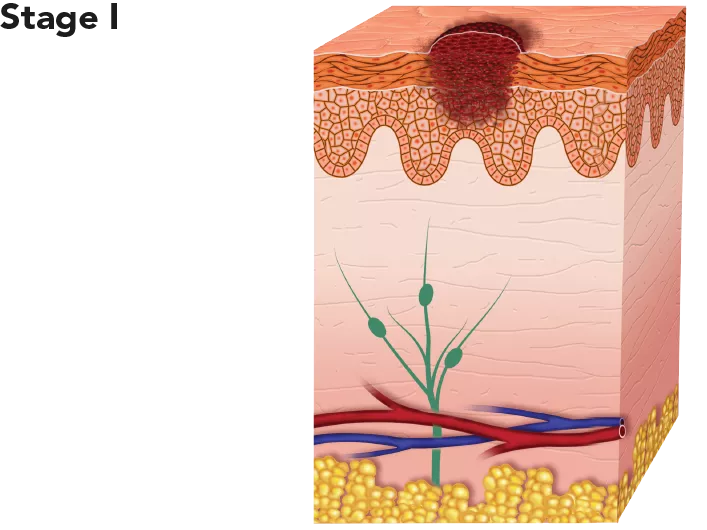
| The tumour is thicker than in Stage 0 and may have grown into the dermis, the dense inner layer of the skin, but is still relatively thin. | With appropriate treatment it is highly curable. Low risk for recurrence or metastasis. 5-year survival rate: 98.4%. |
Image
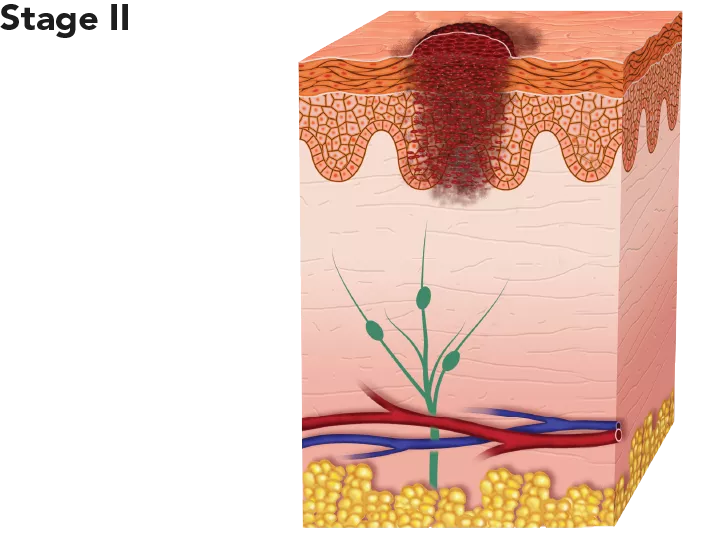
| The tumour is thicker than in Stage I and has grown past the epidermis into the dermis. At this stage, the melanoma has a higher chance of spreading. | With appropriate treatment it is considered intermediate to high risk for recurrence or metastasis. 5-year survival rate: 98.4%. |
Image
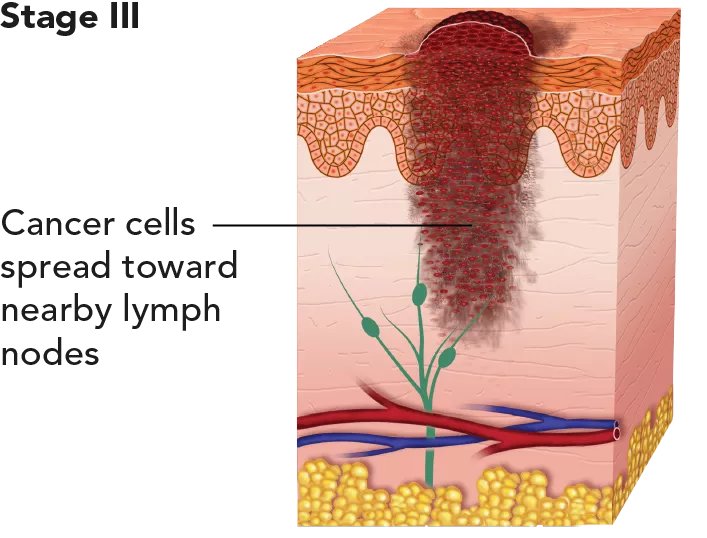
| The cancer has spread to one or more lymph nodes. There may also be ulceration. Stage III melanoma, which cannot be removed by surgery (resection), is classed as unresectable Stage III melanoma. | With appropriate treatment it is considered intermediate to high risk for recurrence or metastasis. 5-year survival rate: 63.6%. |
Image
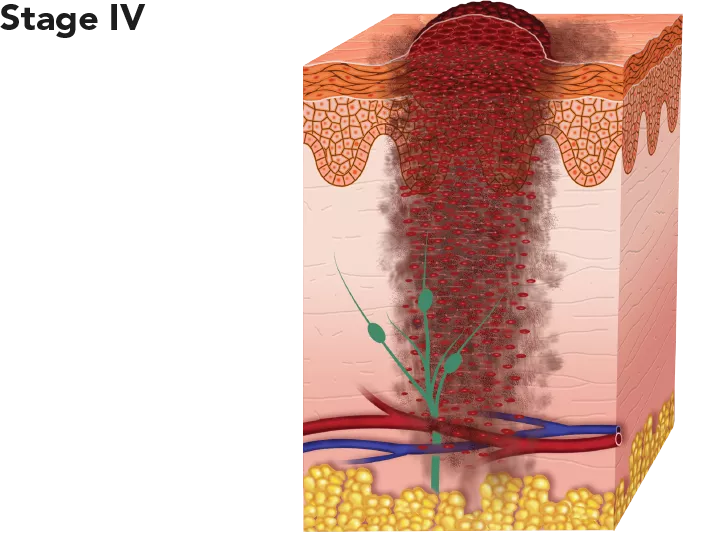
| The cancer has spread to distant lymph nodes and/or other places in the skin or parts of the body, such as the lungs, liver, bones and brain. This stage is also known as metastatic melanoma. | The cancer has already metastasised and is therefore very difficult to cure. However, some patients respond well to treatment and can survive for many years post-diagnosis. 5-year survival rate: 22.5%. |
*Melanoma treatments have improved significantly with the addition of immunotherapy and targeted therapies. These survival rates only partially reflect these advancements.10 | ||
Identification of a BRAF mutation opens up potential options for patients to receive BRAF targeted treatments11
Genetic mutations in many melanomas cause the tumour to grow out of control. The three most common mutations in melanoma are in BRAF, NRAS and c-KIT genes.12 By identifying the mutations that have caused a particular patient’s cancer, a more personalised approach to treatment can be offered. The only mutation for which targeted therapy is available is for the BRAF gene.12
The efficacy and safety of trametinib have not been evaluated in patients whose melanoma tested negative for the BRAF V600 mutation and should not be used in these patients.1,2
The role of the BRAF gene in melanoma
The RAS/RAF/MEK/ERK pathway, also known as the MAP kinase pathway (MAPK), is a critical pathway involved in normal cellular functions, but it may be disrupted in many human cancers, including melanoma.13 This pathway plays a key role in regulating the growth, proliferation, and survival of normal cells, including melanocytes.14–16
Mutation of BRAF leads to activation of the MAPK pathway, which stimulates cellular growth and inhibits pro-apoptotic signals. This drives cellular proliferation in tumour cells, migration, survival, angiogenesis and potential metastasis.13
Schematic of the BRAF pathway in melanoma13
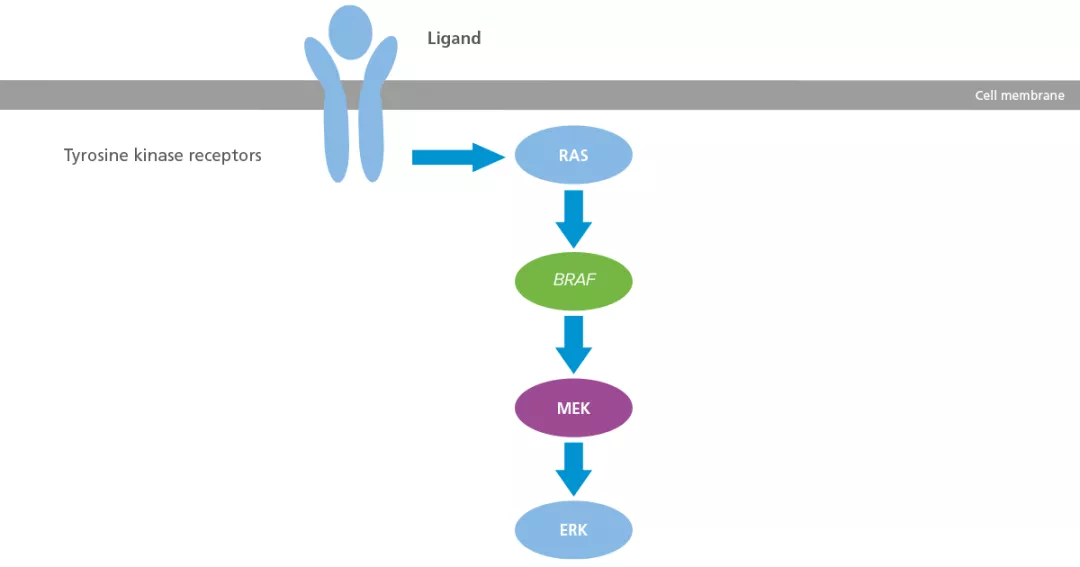
Adapted from Inamdar GS, et al. 2010.13
About 50% of melanomas have a BRAF mutation.14 Among the BRAF mutations in melanoma, >90% are at codon 600 known as BRAF V600 (also known as BRAF V600 mutation-positive or BRAF-positive melanoma).12,13,17 V600E is a specific mutation in the BRAF gene that results in an amino acid substitution at position 600 in the BRAF protein: valine (V) is replaced by glutamic acid (E).13
Several studies showed that patients with a BRAF mutation are diagnosed with metastatic disease at an earlier age than patients with BRAF wild-type.17
Median age at diagnosis of distant metastases17
In a prospective study of Australian patients with metastatic melanoma (n=197), a comprehensive range of clinicopathologic variables were correlated with BRAF mutation status, and a survival analysis was conducted.17

*m+: describes a mutation in the BRAF gene; BRAF WT: describes genes that have no mutations.
Due to the high percentage of melanomas harbouring a BRAF mutation, all melanoma patients should undergo BRAF mutation testing via a biopsy of the tumour to identify if the melanoma is BRAF-positive.11
BRAF mutation testing methods
There are several molecular platforms and methods available to analyse the BRAF mutation status of melanoma tissue, such as first-generation (Sanger) sequencing, polymerase chain reaction (PCR), mass spectrometry base sequencing, next generation sequencing (NGS), high-resolution melting (HRM) analysis, pyrosequencing, and immunohistochemistry.
Each of these methods has different technical and practical advantages and certain limitations, which makes some methods better suited for particular settings and to the workflow in individual laboratories.
One important consideration when selecting the testing methodology is its ability to detect not just the V600E mutation but also other less-common mutations at this location (e.g. V600K and V600D).
Regional biomarker testing centres
In England, BRAF testing can be done as part of the NGS panel. You can find more information in the National Genomic Test Directory for cancer in England.18
A personalised approach to treatment offers patients with melanoma a chance of survival14

Stages 0–II melanoma:
surgery is the main treatment.19

Stage III melanoma:
may require a complete lymph node dissection or lymphadenectomy, followed by adjuvant therapy to reduce the risk of recurrence.19
Stage IV melanoma:
main treatments are targeted therapy, immunotherapy and radiotherapy. Other treatments that are used less frequently include chemotherapy and surgery.4,11
Role of systemic therapies in the treatment of advanced melanoma
A greater understanding of the biology of melanoma has led to the development of drugs targeting underlying mutations, and the development of agents that modulate the immune system.13 The availability of these agents has helped improve the prognosis for patients with advanced melanoma (Stage III to Stage IV).14
Targeted therapies block cancer growth through interaction with specific molecules.20 These therapies may switch-off uncontrolled signalling caused by a specific genetic mutation, or block one or more pathways to inhibit cell proliferation in the cancer cells.20 Targeted therapies are usually administered as tablets or capsules, taken orally, a convenient option that allows for self-administration at home.20 Some targeted therapies may be taken alone or in combination with other targeted therapies.11,21–24
Immunotherapies stimulate the immune system to attack melanoma by recognising and destroying malignant cells. They are used to switch on or block certain aspects of the immune system pathways to improve the body’s natural immune response.19 These drugs are usually given as an intravenous infusion every few weeks in a healthcare setting, either as a single treatment or in combination with other types of treatment.21–24
Chemotherapy destroys cells that are in the process of dividing into two cells, thereby stopping or slowing the growth of tumours. Chemotherapy drugs affect specific stages of a cell’s cycle, but they do not differentiate between malignant and healthy cells that have a rapid cell cycle, such as those in the gastrointestinal tract, bone marrow and hair follicles.25
Milestones in melanoma diagnosis and treatment26–33
Please refer to individual SmPCs for the full licensed indications.
*European approvals.
AJCC, American Joint Committee on Cancer; BRAF V600E/K/D, mutation of the BRAF gene at valine (v) 600 to glutamate (E), lysine (K) or aspartate (D); ERK, extracellular signal-related kinase; GP, general practitioner; HRM, resolution melting: MAPK, mitogen-activated protein kinase; MEK, mitogen-activated extracellular signal-regulated kinase; NGS, next-generation sequencing; PCR, polymerase chain reaction; RAS, rat sarcoma; SmPC, summary of product characteristics; TNM, tumour size, lymph node involvement, and metastases.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Cancer Research UK. Available at: https://www.cancerresearchuk.org/health-professional/cancer-statistics/s... [Accessed March 2025].
Davis LE, et al. Cancer Biol Ther 2019;20:1366–1379.
British Association of Dermatologists. Available at: https://www.skinhealthinfo.org.uk/wp-content/uploads/2020/10/ABCDEasy-Leaflet.pdf [Accessed March 2025].
Keunga EZ and Gershenwalda JE. Expert Rev Anticancer Ther 2018; 8:775–784.
Melanoma Research Alliance. Available at: https://www.curemelanoma.org/about-melanoma/melanoma-staging/ [Accessed March 2025].
American Cancer Society. Available at: https://www.cancer.org/cancer/melanoma-skin-cancer/detection-diagnosis-s... [Accessed March 2025].
National Cancer Institute. SEER Training Modules. Staging. Available at: https://training.seer.cancer.gov/melanoma/abstract-code-stage/staging.html [Accessed March 2025].
Melanoma Research Alliance. Melanoma Survival Rates. Available at:https://www.curemelanoma.org/about-melanoma/melanoma-staging/melanoma-su... [Accessed March 2025].
Michielin O, et al. Ann Oncol 2019;30:1884–1901.
Ponti G, et al. Anticancer Research 2017;37:7043–7048.
Inamdar GS, et al. Biochem Pharmacol 2010;80:624–637.
Ascierto PA, et al. J Transl Med 2012;10:85.
Wellbrock C, et al. Nat Rev Mol Cell Biol 2004;5:875–885.
Wan PTC, et al. Cell 2004;116:855–867.
Long GV, et al. J Clin Oncol 2011;29:1239–1246.
NHS England. Available at: https://www.england.nhs.uk/publication/national-genomic-test-directories/ [Accessed March 2025].
American Cancer Society. Treatment of melanoma skin cancer by stage. Available at: https://www.cancer.org/cancer/types/melanoma-skin-cancer/treating/by-sta... [Accessed March 2025].
Cancer Australia. Available at: https://www.canceraustralia.gov.au/impacted-cancer/treatment/targeted-therapy [Accessed March 2025].
ESMO. Available at: https://www.esmo.org/for-patients/personalised-medicine-explained/melanoma [Accessed March 2025].
National Institute for Health and Care Excellence (NICE) 2015. https://www.nice.org.uk/guidance/ng14/chapter/1-Recommendations#managing... [Accessed March 2025].
Zhang J, et al. Cancer Lett 2024;586:216633.
Marsden JR, et al. Br J Dermatol 2010;163:238–256.
Cancer Research UK. Available at: https://www.cancerresearchuk.org/about-cancer/cancer-in-general/treatmen... [Accessed March 2025].
Rebecca VW, et al. Melanoma Res 2012;22:114–122.
European Medicines Agency (EMA) search. Available at: https://www.ema.europa.eu/en/search/search?search_api_views_fulltext=Mel... [Accessed March 2025].
European Medicines Agency. Assessment Report for YERVOY (ipilimumab). Available at: https://www.ema.europa.eu/en/documents/assessment-report/yervoy-epar-pub... [Accessed March 2025].
European Medicines Agency. Assessment Report ZELBORAF (vemurafenib). Available at: https://www.ema.europa.eu/en/documents/assessment-report/zelboraf-epar-p... [Accessed March 2025].
European Medicines Agency. CHMP Assessment Report. TAFINLAR (dabrafenib). Available at: https://www.ema.europa.eu/en/documents/assessment-report/tafinlar-epar-p... [Accessed March 2025].
European Medicines Agency. CHMP Assessment Report. MEKINIST (trametinib). Available at: https://www.ema.europa.eu/en/documents/assessment-report/mekinist-epar-p... [Accessed March 2025].
European Medicines Agency. KEYTRUDA (pembrolizumab). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/keytruda [Accessed March 2025].
European Medicines Agency. OPDUALAG (relatlimab/nivolumab). Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/opdualag [Accessed March 2025].
UK | March 2025 | FA-11218014
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

