

COMBI-AD safety
TAFINLAR® (dabrafenib) in combination with MEKINIST® (trametinib) is indicated in adult patients with unresectable or metastatic melanoma with a BRAF V600 mutation.1,2
TAFINLAR in combination with MEKINIST is indicated for the adjuvant treatment of adult patients with Stage III melanoma with a BRAF V600 mutation, following complete resection.1,2
Common adverse events include:
TAFINLAR + MEKINIST: The most common adverse reactions (incidence ≥20%) for dabrafenib in combination with trametinib were pyrexia, fatigue, nausea, chills, headache, diarrhoea, vomiting, arthralgia and rash1,2
TAFINLAR: The most common adverse reactions (incidence >15%) reported with dabrafenib were hyperkeratosis, headache, pyrexia, arthralgia, fatigue, nausea, papilloma, alopecia, rash and vomiting1
MEKINIST: The most common adverse reactions (incidence ≥20%) for trametinib were rash, diarrhoea, fatigue, oedema peripheral, nausea and dermatitis acneiform2
For more safety information on TAFINLAR and MEKINIST, click here.
For the full safety profile, please refer to the Summary of Product Characteristics (SmPC) for TAFINLAR and MEKINIST.
Adverse event reporting: Details of how to report adverse events are available at the bottom of the page. Please refer to the respective SmPC for all licensed indications.
The safety profile of TAFINLAR + MEKINIST is well established1–3
TAFINLAR + MEKINIST in the adjuvant setting has a well-established safety profile.3
Key safety profile insights
The most common adverse events (AEs) observed with TAFINLAR + MEKINIST in the COMBI-AD study included: pyrexia, fatigue and nausea.4
No safety analysis was performed at the 5-year follow-up of COMBI-AD, as no new safety data were generated since the primary analysis when the last patient was treated on 1 December 2015.1,4
In general, the AE profile was consistent between the adjuvant and metastatic settings (COMBI-AD vs COMBI-v and COMBI-d)5,6
All scheduled doses were completed by 63% of patients (272 of 435) on TAFINLAR, 64% of patients (277 of 435) on MEKINIST, and 53% of patients (227 of 432) on placebo4
The majority of patients completed the scheduled 12 months of TAFINLAR + MEKINIST combination with a median dose that was close to the scheduled dose for each drug. Less than one third (26%) of the patients treated with TAFINLAR + MEKINIST discontinued treatment because of an AE4
The peak onset of all AEs occurring in ≥15% of patients in the TAFINLAR + MEKINIST arm occurred within the first 3 months of treatment7
Frequencies of dose reductions or dose interruptions decreased as duration of treatment increased7
AE data
AEs in the COMBI-AD study4,8
TAFINLAR + MEKINIST* (n=435) | Placebo* (n=432) | |
|---|---|---|
| AE category, n (%) | All grades | All grades |
| Any AE | 422 (97) | 380 (88) |
| AEs related to study drug | 398 (91) | 272 (63) |
| Any Grade 3/4 AE | 180 (41) | 61 (14) |
| Any SAE | 155 (36) | 44 (10) |
| SAEs related to study drug | 117 (27) | 17 (4) |
| Fatal AEs related to study drug | 0 | 0 |
| AEs leading to dose interruption | 289 (66) | 65 (15) |
| AEs leading to dose reduction | 167 (38) | 11 (3) |
| AEs leading to treatment discontinuation | 114 (26) | 12 (3) |
*Safety population.
Adapted from Long GV, et al. 20174 and Hauschild A, et al. 2017.8
Most common AEs
Most common AEs (>20% of patients) in the COMBI-AD study4,8
TAFINLAR + MEKINIST (n=435) | Placebo (n=432) | |||
| AEs, n (%) | All grades | Grade 3/4 | All grades | Grade 3/4 |
| Any grade | 422 (97) | 180 (41) | 380 (88) | 61 (14) |
| Pyrexia | 273 (63) | 23 (5) | 47 (11) | 2 (<1) |
| Fatigue | 204 (47) | 19 (4) | 122 (28) | 1 (<1) |
| Nausea | 172 (40) | 4 (1) | 88 (20) | 0 |
| Headache | 170 (39) | 6 (1) | 102 (24) | 0 |
| Chills | 161 (37) | 6 (1) | 19 (4) | 0 |
| Diarrhoea | 144 (33) | 4 (1) | 65 (15) | 1 (<1) |
| Vomiting | 122 (28) | 4 (1) | 43 (10) | 0 |
| Arthralgia | 120 (28) | 4 (1) | 61 (14) | 0 |
| Rash | 106 (24) | 0 | 47 (11) | 1 (<1) |
Eleven patients (3%) in the TAFINLAR + MEKINIST arm and 10 patients (2%) in the placebo arm had new primary melanoma; 8 (2%) and 7 (2%), respectively, had cutaneous squamous-cell carcinoma/keratoacanthoma; 19 (4%) and 14 (3%), respectively, had basal cell carcinoma; and 10 (2%) and 4 (1%), respectively, had non-cutaneous cancers.
Adapted from Long GV, et al. 2017.4
Pyrexia was the most common AE associated with TAFINLAR + MEKINIST treatment in the adjuvant setting of patients with Stage III melanoma. The majority of pyrexia events were Grade 1 or 2.4
TAFINLAR/MEKINIST when used as monotherapy, and both TAFINLAR and MEKINIST when used in combination, should be interrupted if the patient's temperature is ≥38°C. In case of recurrence, therapy can also be interrupted at the first symptom of pyrexia. Treatment with antipyretics such as ibuprofen or acetaminophen/paracetamol should be initiated. The use of oral corticosteroids should be considered in those instances in which antipyretics are insufficient. Patients should be evaluated for signs and symptoms of infection. TAFINLAR/MEKINIST when used as monotherapy or both TAFINLAR and MEKINIST when used in combination, should be restarted if the patient is symptom-free for at least 24 hours either (1) at the same dose level, or (2) reduced by one dose level if pyrexia is recurrent and/or was accompanied by other severe symptoms including dehydration, hypotension or renal failure. Please see individual SmPCs for further information.1,2
First occurrence of AEs
The first occurrence of AEs was generally in the first 3 months of patients receiving adjuvant TAFINLAR + MEKINIST.7 The peak onset of AEs occurring in ≥15% of patients was also within the first 3 months of starting treatment.7
First occurence of AEs in the COMBI-AD study*7
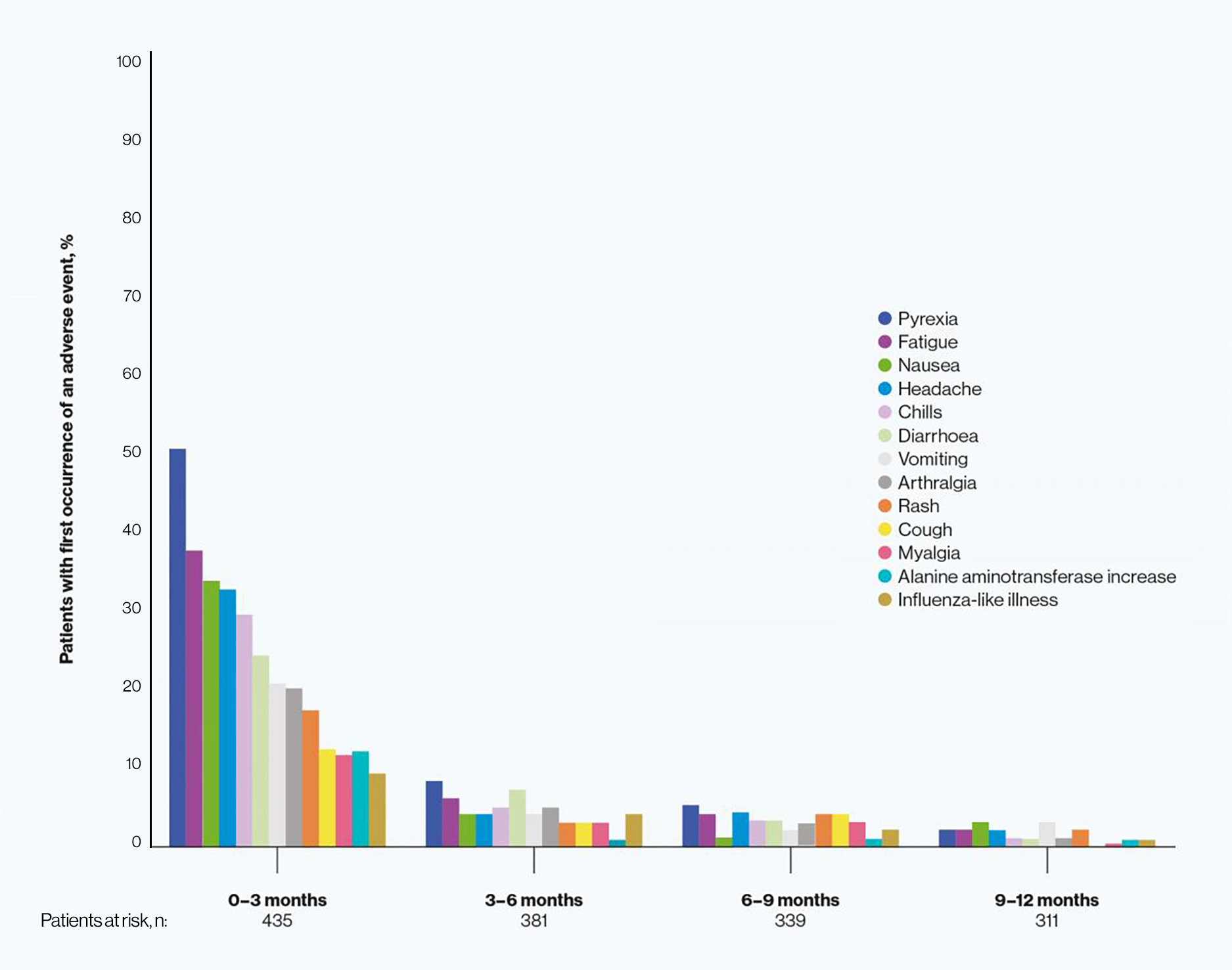
*In patients who received TAFINLAR + MEKINIST.
Adapted from Atkinson A, et al. 2018.7
A pooled analysis of pyrexia from patients treated with TAFINLAR + MEKINIST
Data from four clinical trials (N=1076) were used for the largest analysis of BRAF inhibitor + MEK inhibitor-induced pyrexia5,9
Across four TAFINLAR + MEKINIST clinical trials (n=1076),* 39% of patients treated with TAFINLAR + MEKINIST did not experience a pyrexia event, while 32% of patients experienced only one or two events5,9
In total, 39% of patients treated with TAFINLAR + MEKINIST did not experience a pyrexia event, while 32% of patients experienced only one or two events5,9
The majority of pyrexia events were mild or moderate (Grade 1/2). Only 9% of patients treated with TAFINLAR + MEKINIST experienced Grade 3 events and <1% of patients experienced Grade 4 events5,9
Most pyrexia events were completely resolved with either temporary dose interruption or no dose change5,9
Only 6% of patients discontinued treatment with TAFINLAR or MEKINIST due to pyrexia5,9
Pooled analysis of pyrexia: Single vs multiple occurrences*5
These data are based on a pooled analysis of pyrexia occurrence across four clinical trials* representing the largest analysis of a BRAF inhibitor + MEK inhibitor-induced pyrexia to date.5
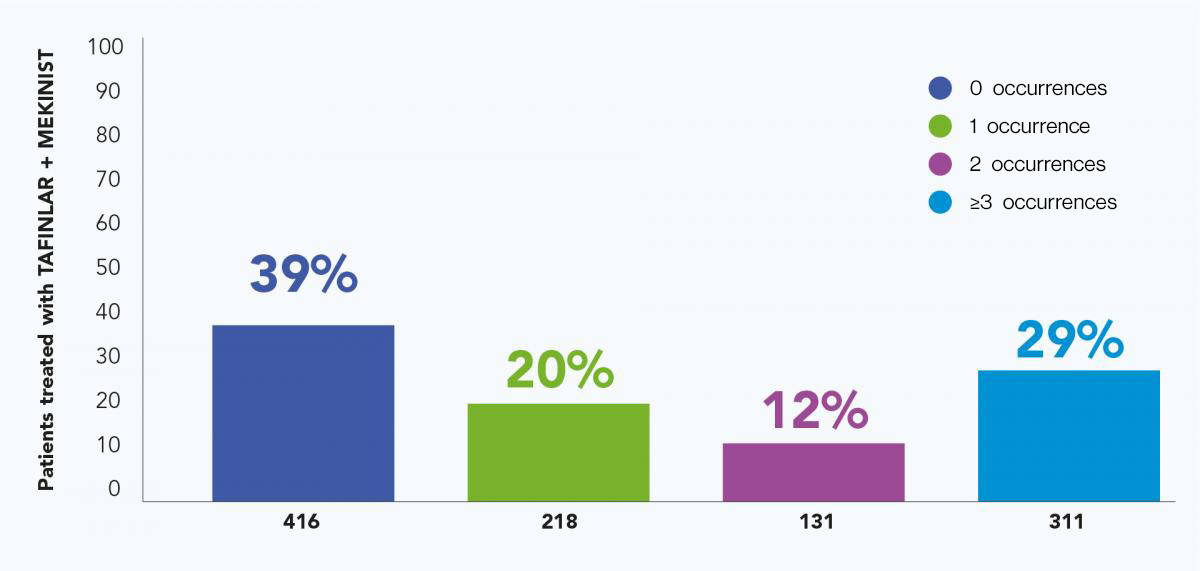
*Pyrexia was analysed from the following clinical trials in melanoma and in metastatic non-small cell lung cancer (NSCLC): Registrational Phase II trial (NCT01336634) in metastatic NSCLC (n=82); COMBI-AD in resected Stage III melanoma (n=435); COMBI-d in unresectable or metastatic melanoma (n=209); COMBI-v in unresectable or metastatic melanoma (n=350).4–6,9,10
Adapted from Robert C, et al. 2019.5
Please visit the management of adult patients with BRAF V600-positive melanoma page for further information on monitoring for, and managing, pyrexia in patients receiving TAFINLAR + MEKINIST.
AE, adverse event; BRAF V600, mutation of the BRAF gene at valine (V) 600; NSCLC, non-small cell lung cancer; SAE, serious adverse event; SmPC, summary of product characteristics.
References
TAFINLAR (dabrafenib) Summary of Product Characteristics.
MEKINIST (trametinib) Summary of Product Characteristics.
Dummer R, et al. N Engl J Med 2020; DOI: 10.1056/NEJMoa2005493.
Long GV, et al. N Engl J Med 2017;377:1813–1823.
Robert C, et al. Presented at ESMO 2019;27 September – 1 October, Barcelona, Spain.
Robert C, et al. N Engl J Med 2019;381:626–636.
Atkinson V, et al. Presented at ESMO 2018;19–23 October, Munich, Germany.
Hauschild A, et al. Presented at ESMO 2017;8–12 September, Madrid, Spain.
Schadendorf D, et al. Eur J Cancer 2021;153:234–241.
Planchard D, et al. J Thorac Oncol 2022;17:103–115
UK | April 2025 | FA-11218799
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

