Prescribing information (external link)


Patients with visceral metastases
Indications1
Advanced breast cancer (aBC)
KISQALI® (ribociclib) is indicated for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor (AI) or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy (ET).
In pre/perimenopausal women, the ET should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.
For more information on the safety profile in aBC, click here.
Please consult your local Summary of Product Characteristics (SmPC) for the full safety profile.
Use the buttons below to explore the data for KISQALI in eligible patients with visceral metastases.
All-grade neutropenia (59.3% vs 64.3%), nausea (45.1% vs 49.2%), diarrhoea (33.8% vs 32.4%), fatigue (30.5% vs 35.4%) and arthralgia (30.2% vs 42.0%) rates were similar in in patients in the two groups, respectively
Rates of grade 3/4 alanine transaminase (ALT; 7.3% vs 9.9%) and aspartate aminotransferase (AST; 7.6% vs 5.5%) elevations were similar in in patients in the two groups, respectively
Discontinuation due to treatment-related AEs was numerically higher with combo CT vs KISQALI + ET (27.0% vs 6.3%, respectively)4
Higher rates of haematologic events including neutropenia and leukopenia were observed with KISQALI + ET while higher rates of non-haematologic events including nausea, vomiting, diarrhoea and fatigue were observed with combo CT4
No new safety signals were observed in patients treated with KISQALI + ET4
1L KISQALI + ET has been studied in patients with visceral metastases2
Special warning/precaution for use: the efficacy and safety of KISQALI have not been studied in patients with critical visceral disease.1
Pooled exploratory analysis of the MONALEESA trials: N=1889, pooled exploratory analysis of KISQALI + ET vs placebo + ET in patients with HR+/HER2– aBC enrolled in the MONALEESA-2, -3 and -7 trials. Median progression-free survival (mPFS) and median overall survival (mOS) were evaluated in a pooled population of patients with visceral metastases, liver metastases and visceral metastases ≥3 sites. The same analysis was conducted in 1L patients separately. 59.5% (n=1124) of the total population had visceral metastases, 640 patients in the KISQALI arm vs 484 in the placebo arm. 1229 patients received 1L therapy, of these patients 57.7% (n=709) had visceral metastases.2
Patient sub-groups:2

Visceral metastases
57.7% of patients receiving 1L treatment (n=709/1229)

Liver metastases
20.8% of patients receiving 1L treatment (n=256/1229)

Visceral metastases ≥3 sites
36.4% of patients receiving 1L treatment (n=447/1229)
ET was defined as AI or fulvestrant and LHRH.3
KISQALI is not recommended to be used in combination with tamoxifen.1
For individual MONALEESA study designs, please see the bottom of the page.
In this pooled exploratory analysis, a longer mOS was observed with 1L KISQALI + ET in HR+/HER2– aBC patients with visceral metastases vs placebo + ET2,3
mOS in the 1L treatment subgroup (n=709; median follow-up: 72 months)2
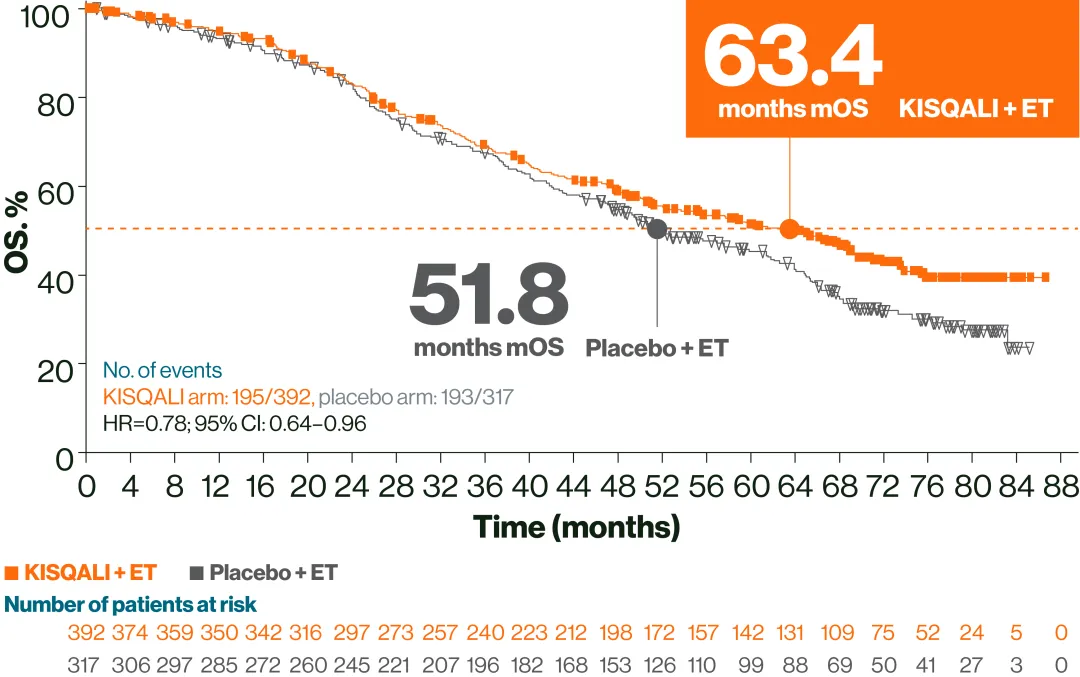
Adapted from Yardley DA, et al. 2022.2
These data are exploratory and hypothesis-generating only.
KISQALI showed a consistent safety profile in patients with or without visceral metastases2
Adverse events (AEs, ≥30%) in patients with or without visceral metastases in the KISQALI + ET arm
| With visceral metastases (n=639) | Without visceral metastases (n=426) | ||
All grades, n (%) | Grade 3/4, n (%) | All grades, n (%) | Grade 3/4, n (%) | |
Neutropenia | 397 (62.1) | 320 (50.1) | 249 (58.5) | 200 (46.9) |
Nausea | 303 (47.4) | 15 (2.3) | 194 (45.5) | 3 (0.7) |
Arthralgia | 236 (36.9) | 7 (1.1) | 162 (38.0) | 5 (1.2) |
Fatigue | 213 (33.3) | 11 (1.7) | 162 (38.0) | 12 (2.8) |
Diarrhoea | 211 (33.0) | 13 (2.0) | 146 (34.3) | 7 (1.6) |
Adapted from Yardley DA, et al. 2022.2
Rates of AEs, including liver enzyme elevations, were similar in patients with or without liver metastases with KISQALI + ET vs placebo + ET:2
Special warning/precaution for use: Liver function tests (LFTs) should be performed before initiating treatment with KISQALI. After initiating treatment, liver function should be monitored. Based on the severity of the transaminase elevations, treatment with KISQALI may have to be interrupted, reduced or discontinued as described in Table 3 of the SmPC. Recommendations for patients who have elevated AST/ALT grade≥3 at baseline have not been established.1 |
The most common adverse drug reactions (ADRs) (reported at a frequency ≥20%) in the dataset for which the frequency for KISQALI + AI exceeds the frequency for AI alone were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests.1
The most common grade 3/4 ADRs (reported at a frequency of ≥2%) in the pooled dataset for which the frequency for KISQALI + any combination exceeds the frequency for placebo + any combination were neutropenia, leukopenia, abnormal LFTs, lymphopenia, infections, back pain, anaemia, fatigue, hypophosphataemia and vomiting.1
Dose reduction due to AEs, regardless of causality, occurred in 39.5% of patients receiving KISQALI in the Phase III clinical studies regardless of the combination. Permanent discontinuation was reported in 8.7% of patients receiving KISQALI and any combination in the Phase III clinical studies.1
Please refer to the SmPC for the full safety profile.
Discover the QoL data in patients treated with KISQALI + ET
Final analysis of the Phase II RIGHT CHOICE trial of 1L KISQALI + ET vs combination CT in premenopausal women with HR+/HER2–aBC and clinically aggressive visceral metastases4
Special warning/precaution for use:
Critical visceral disease
The efficacy and safety of KISQALI have not been studied in patients with critical visceral disease.
RIGHT Choice: N=222, open-label, multicentre, 1:1 randomised, Phase II trial, in pre/perimenopausal women with clinically aggressive HR+/HER2− aBC (including symptomatic visceral metastases, rapid disease progression or impending visceral compromise or markedly symptomatic non-visceral disease). 1L KISQALI (600 mg daily; 3 weeks on, 1 week off) + AI and goserelin or investigator’s choice of combination chemotherapy (combo CT, docetaxel plus capecitabine, paclitaxel plus gemcitabine, or capecitabine plus vinorelbine). The primary endpoint was PFS and secondary endpoints included time to treatment failure (TTF), 3-month TTF, ORR, CBR, time to treatment response, OS, frequency/severity of AEs, change from baseline in global health status/QoL scale score and 3-month treatment failure rate.4,5
Patient subgroups:4

Symptomatic visceral metastases
66% of KISQALI patients (n=74)
69% of combo CT patients (n=76)
A mPFS benefit was observed with KISQALI + ET vs combo CT in patients with clinically aggressive disease4
Special warning/precaution for use: the efficacy and safety of KISQALI have not been studied in patients with critical visceral disease.1
RIGHT Choice PFS analysis; median follow-up: 72 months4

Adapted from Lu Y-S, et al. 2024.4
ET was defined as AI and LHRH.4
KISQALI is not recommended to be used in combination with tamoxifen.1
Lower rates of symptomatic AEs were observed with KISQALI + ET vs combo CT4
AEs (≥20%) in either arm4
| KISQALI + ET (n=112) | Combo CT (n=100) | ||
All grades | Grade 3/4 | All grades | Grade 3/4 | |
Any AEs, n (%) | 112 (100) | 89 (79.5) | 100 (100) | 73 (73) |
Haematologic AEs | ||||
Neutropenia | 94 (84) | 67 (60) | 50 (50) | 36 (36) |
Leukopenia | 55 (49) | 28 (25) | 26 (26) | 8 (8) |
Anaemia | 40 (36) | 6 (5) | 43 (43) | 11 (11) |
Non-haematologic AEs | ||||
Elevated ALT | 23 (21) | 6 (5) | 30 (30) | 6 (6) |
Elevated AST | 23 (21) | 8 (7) | 29 (29) | 5 (5) |
Nausea | 14 (13) | 0 | 27 (27) | 1 (1) |
Alopecia | 12 (11) | 0 | 20 (20) | 0 |
Vomiting | 8 (7) | 1 (1) | 30 (30) | 0 |
Diarrhoea | 3 (3) | 0 | 26 (26) | 1 (1) |
Fatigue | 9 (8) | 0 | 25 (25) | 2 (2) |
Palmar-plantar erythrodysaesthesia | 3 (3) | 0 | 32 (32) | 5 (5) |
Adapted from Lu Y-S, et al. 2024.4
Ten patients randomised to the combo CT arm were not included in the safety set as they did not receive any study treatment after withdrawal of consent following knowledge of randomisation to the CT arm (n=9) and withdrawal based on investigator’s decision (n=1).4
Want to learn more about KISQALI + ET in patients with HR+/HER2– aBC?
Use the buttons below to explore the data in that patient subgroup.
Study designs
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, Phase III trial in postmenopausal women with HR+/HER2− aBC. As 1L in advanced disease. No prior ET for aBC and no previous systemic chemotherapy for advanced disease. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (2.5 mg continuous). The primary endpoint was locally assessed progression-free survival (PFS) and the key secondary endpoint was OS. Other secondary endpoints included the overall response rate (ORR, complete or partial response), clinical benefit rate (CBR, overall response plus stable disease lasting 24 weeks or more), safety, and quality of life (QoL) assessments.6
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, Phase III trial. As 1L and 2L in advanced disease plus those with early relapse in postmenopausal women with HR+/HER2– aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. The primary endpoint was locally assessed PFS. Secondary endpoints included OS, ORR, CBR, and safety and tolerability. No prior ET or after disease progression on 1L ET for aBC.7
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, Phase III trial in pre/perimenopausal women with HR+/HER2− aBC. As 1L in advanced disease and in patients who received 1 or fewer lines of chemotherapy for aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + NSAI or tamoxifen 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on Day 1 of every cycle). The primary endpoint was investigator assessed PFS. The key secondary endpoint was OS, defined as the time from randomisation to death from any cause. The other secondary endpoints included: proportion of patients who achieved an objective response, clinical benefit, time to treatment response, duration of response, time to definitive deterioration of ECOG PS from baseline, time to 10% deterioration for EORTC QLQ-C30 and safety.8
KISQALI is not recommended to be used in combination with tamoxifen.1
1L, first-line; aBC, advanced breast cancer; ADR, adverse drug reaction; AE, adverse event; AI, aromatase inhibitor; ALT, alanine transaminase; AST, aspartate aminotransferase; CBR, clinical benefit rate; CI, confidence interval; combo CT, combination chemotherapy; ECOG PS, Eastern Cooperative Oncology Group performance status; EORTC QLQ-C30, European Organisation for the Research and Treatment of Cancer quality of life questionnaire; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; LFT, liver function test; LHRH, luteinising hormone-releasing hormone; mOS, median overall survival; mPFS, median progression-free survival; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QoL, quality of life; SmPC, summary of product characteristics; TTF, time to treatment failure.
References
KISQALI® (ribociclib) Summary of Product Characteristics.
Yardley DA, et al. Poster 205P. European Society for Medical Oncology Congress. 9–13 September 2022; Paris, France.
Yardley DA, Ann Oncol 2022;33(S7):S2629.
Lu Y-S, et al. J Clin Oncol 2024; 42(23):2812–2821.
ClinicalTrials.gov. Study to compare the combination of ribociclib plus goserelin acetate with hormonal therapy versus combination chemotherapy in premenopausal or perimenopausal patients with advanced or metastatic breast cancer (RIGHT Choice). Available at: https://www.clinicaltrials.gov/study/NCT03839823 [Accessed December 2025].
Hortobagyi GN, et al. N Engl J Med 2016;375:1738–1748.
Slamon DJ, et al. J Clin Oncol 2018;36(24):2465–2472.
Tripathy D, et al. Lancet Oncol 2018;109:904–915.
UK | December 2025 | FA-11576568
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

