Check your patients’ eligibility
Click to use our interactive tool to assess whether your patients with HR+/HER2– eBC could be eligible for treatment with KISQALI + AI
UK | January 2026 | FA-11576744
This page is intended for UK healthcare professionals and other relevant decision makers only. If you are a member of the public, please click here.
This portal is funded and owned by Novartis Pharmaceuticals UK Ltd and includes content approved by Novartis.
Adverse events reporting information can be found in the footer of this page.
Early breast cancer (eBC)
KISQALI in combination with an aromatase inhibitor (AI), is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative eBC at high risk of recurrence (see section 5.1 of the SmPC for selection criteria)
In pre- or perimenopausal women, or in men, the AI should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
Advanced breast cancer (aBC)
KISQALI is indicated for the treatment of women with HR+/HER2– locally advanced or metastatic breast cancer in combination with an AI or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy (ET)
In pre- or perimenopausal women, the ET should be combined with an LHRH agonist
KISQALI is not recommended to be used in combination with tamoxifen.
In eBC, KISQALI should be used in combination with an AI (taken orally once daily continuously throughout the 28-day cycle). In aBC, KISQALI should be used in combination with an AI (taken orally once daily continuously throughout the 28-day cycle) or fulvestrant (administered intramuscularly on Days 1, 15 and 29, and once monthly thereafter).1
Please refer to the Summary of Product Characteristics (SmPC) of the co-administered AI, fulvestrant or LHRH for dosing, dose modification guidelines and other relevant safety information in the event of toxicity.
Please refer to the KISQALI SmPC for full information on dosing and administration.
For eBC, the recommended dose is KISQALI 400 mg (two 200 mg film-coated tablets) once daily for 21 consecutive days, followed by 7 days off treatment, resulting in a complete cycle of 28 days.1
For aBC, the recommended dose is KISQALI 600 mg (three 200 mg film-coated tablets) once daily for 21 consecutive days, followed by 7 days off treatment, resulting in a complete cycle of 28 days.1






Please use the arrows to navigate through the slideshow.






Please use the arrows to navigate through the slideshow.
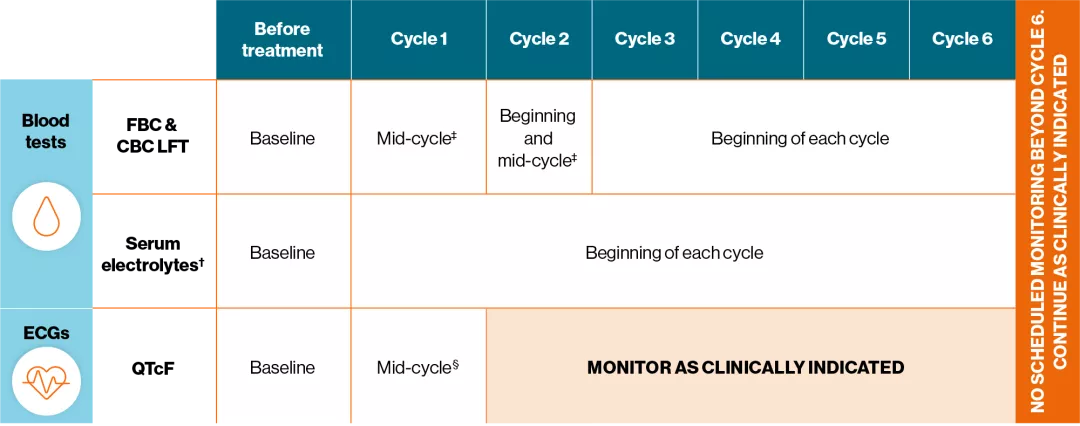
Adapted from KISQALI® (ribociclib) Summary of Product Characteristics.1
†Includes potassium, calcium, phosporus and magnesium.1
‡After initiating treatment, assess every 2 weeks for the first 2 cycles.1
§After initiating treatment, assess on Day 14 of cycle 1.1
KISQALI is primarily metabolised by CYP3A4. Certain medicines can interact with CYP3A4 enzyme activity and may alter the pharmacokinetics of KISQALI. Additionally, KISQALI is a strong CYP3A4 inhibitor at the 600 mg dose and a moderate CYP3A4 inhibitor at the 400 mg dose. Thus, KISQALI may interact with medicinal products which are metabolised via CYP3A4, which may lead to increased serum concentrations of CYP3A4 substrates.1
When KISQALI is co-administered with other medicinal products, the SmPC of the other medicinal products must be consulted for the recommendations regarding co-administration with CYP3A4 inhibitors. Caution is recommended in case of concomitant use with sensitive CYP3A4 substrates with a narrow therapeutic index and the SmPC of the other product should be consulted for the recommendations regarding co-administration with CYP3A4 inhibitors. Thus KISQALI may interact with medicinal products which are metabolised via CYP3A4 which may lead to increased serum concentrations of CYP3A4 substrates.1
Category1 | Examples (including, but not limited to)1 |
Strong CYP3A4 inhibitors Should be avoided
No clinical data with this dose adjustment are available. If dose reduction below 200 mg/day is required, KISQALI treatment should be interrupted. Due to inter-patient variability, the recommended dose adjustments may not be optimal in all patients, therefore close monitoring for KISQALI-related adverse reactions is recommended. In the event of KISQALI-related toxicity, the dose should be modified or treatment should be interrupted until toxicity is resolved. | Clarithromycin, telithromycin, nefazodone, itraconazole, ketoconazole, posaconazole, voriconazole, verapamil, grapefruit, grapefruit juice, indinavir, lopinavir, nelfinavir, ritonavir, saquinavir, telaprevir |
Moderate CYP3A4 inhibitors No dose adjustments of KISQALI are required at initiation of treatment with mild or moderate CYP3A4 inhibitors; however, monitoring of KISQALI-related adverse reactions is recommended. |
|
Strong CYP3A4 inducers Strong CYP3A4 inducers should be avoided.
| Rifampicin, carbamazepine, phenytoin, St. John’s Wort (Hypericum perforatum) |
Moderate CYP3A4 inducers Concomitant use of moderate CYP3A4 inducers may lead to decreased KISQALI exposure and consequently a risk of impaired efficacy, in particular in patients treated with KISQALI at 400 mg or 200 mg once daily. | |
CYP3A4 substrates Caution is recommended with sensitive CYP3A4 substrates with a narrow therapeutic index. | Ciclosporin, sirolimus, tacrolimus, everolimus, alfentanil, fentanyl |
Concomitant administration of KISQALI with certain CYP3A4 substrates should be avoided.
| Alfuzosin, amiodarone, cisapride, pimozide, quinidine, ergotamine, dihydroergotamine, quetiapine, lovastatin, simvastatin, sildenafil, midazolam and triazolam |
Antiarrhythmics and medicines known to prolong QT interval Should be avoided. | Amiodarone, disopyramide, procainamide, quinidine, sotalol; azithromycin, ciprofloxacin, clarithromycin, levofloxacin, moxifloxacin, chloroquine, halofantrine, haloperidol, pimozide, bepridil, methadone, ondansetron (IV) |
Drug transporters Caution and monitoring for toxicity are advised with concomitant administration with sensitive substrates of transporters which exhibit a narrow therapeutic index. | Digoxin, pravastatin, rosuvastatin, pitavastatin, metformin |
Patients should be instructed to avoid grapefruit or grapefruit juice. These are known to inhibit cytochrome CYP3A4 enzymes and may increase the exposure to KISQALI.1
Drug-drug interaction studies between KISQALI and oral contraceptives have not been conducted.1
Co-administration of KISQALI with medicinal products that elevate the gastric pH has not been evaluated in a clinical study.1
For a list of medications and components that are known to interact with CYP3A4 enzyme activity and basic heart function, please see the SmPC.
Please refer to the SmPC for important safety information.1
aBC, advanced breast cancer; AE, adverse event; AI, aromatase inhibitor; Cl, confidence interval; CYP3A4, cytochrome P450 3A4; eBC, early breast cancer; ECG, electrocardiogram; ET, endocrine therapy; FBC, full blood count; HER2−, human epidermal growth factor receptor-2 negative; HR, hazard ratio; HR+, hormone receptor-positive; IV, intravenous; LFT, liver function test; LHRH, luteinising hormone-releasing hormone; mOS, median overall survival; OS, overall survival; QTcF, corrected QT interval by Fridericia's formula; SmPC, summary of product characteristics.
References
KISQALI (ribociclib) Summary of Product Characteristics.
Hart, L. et al. Poster 1017. ASCO Annual Meeting. 3–7 June 2022, Chicago USA.
De Laurentiis, M et al. Poster 311P. European Society for Medical Oncology Virtual Congress. 19–21 September 2020.
UK | July 2025 | FA-11437665
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.