KISQALI® (ribociclib) quality of life
Indications:1
KISQALI is indicated for the treatment of women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative locally advanced or metastatic breast cancer in combination with an aromatase inhibitor or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy
In pre- or perimenopausal women, the endocrine therapy should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.
For more safety information, click here. For the full safety profile, please refer to the KISQALI Summary of Product Characteristics.
Measuring QoL
The European Organisation for Research and Treatment of Cancer QoL questionnaire (EORTC QLQ-C30) is one of the most widely used patient-reported outcome measures in cancer patients. It is comprised of 30 questions scored into 15 scales and assesses the impact on physical, psychological and social functions.7
Key HRQoL results from Phase III studies






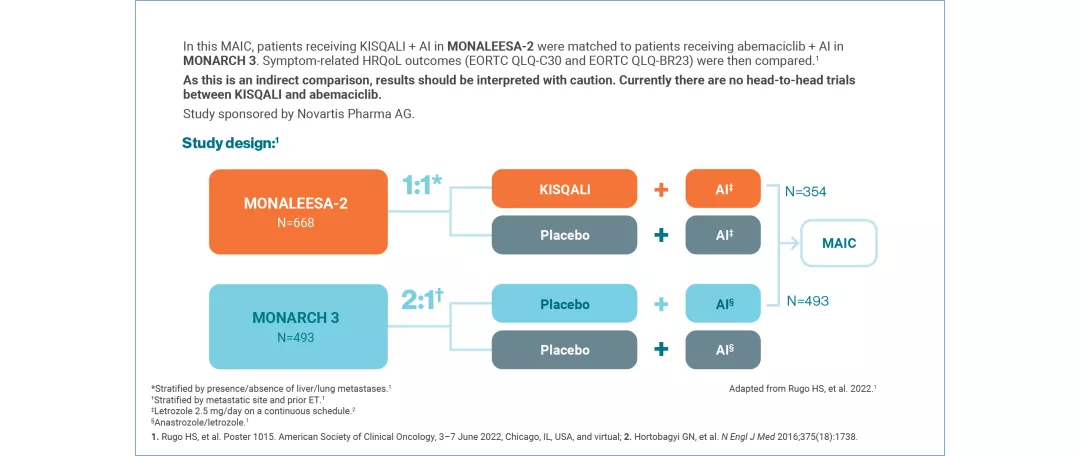
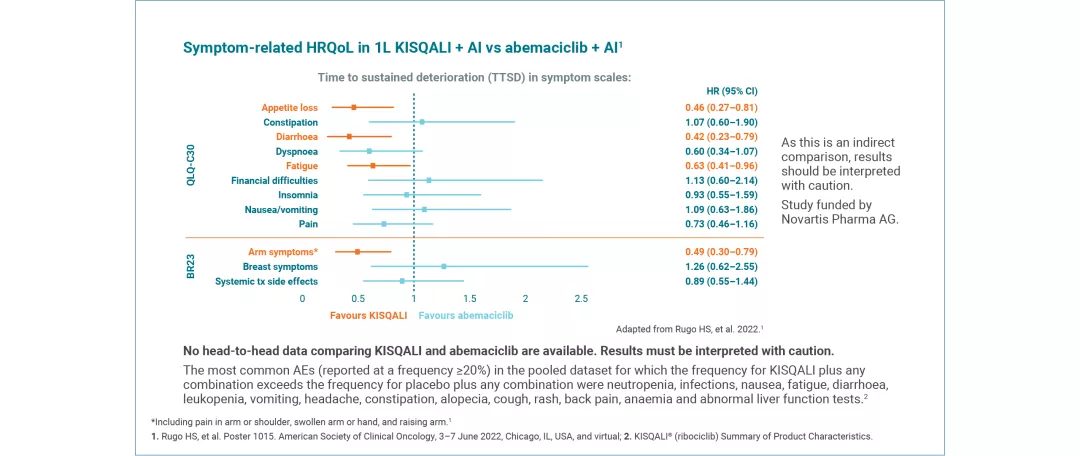
HRQoL versus abemaciclib: Indirect comparison
Where there is a lack of head-to-head data, the MAIC method allows patients from one study to be matched to another, allowing indirect comparison of outcomes, including HRQoL, for the interventions assessed.8



Maintaining QoL is one of the most important considerations for patients with cancer2
HRQoL with KISQALI + ET | |
KISQALI + AI (MONALEESA-2) | MAINTAINED vs placebo + AI |
KISQALI + fulvestrant (MONALEESA-3) | MAINTAINED vs placebo + fulvestrant |
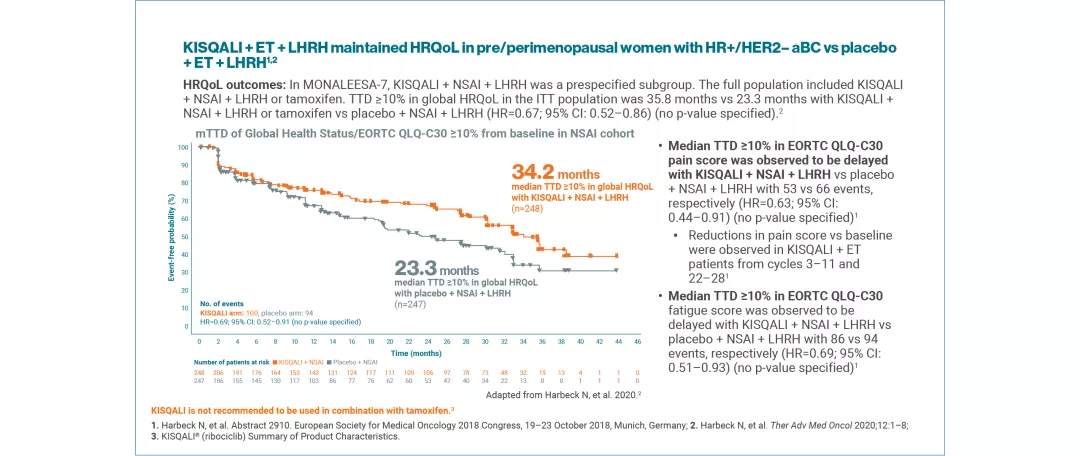
KISQALI + NSAI + LHRH (MONALEESA-7) | MAINTAINED vs placebo + NSAI + LHRH The full population included KISQALI + NSAI + LHRH or tamoxifen. HRQoL (median TTD ≥10%) in the ITT population was higher with KISQALI + NSAI + LHRH or tamoxifen than placebo + NSAI + LHRH or tamoxifen: 35.8 months vs 23.3 months, respectively (HR=0.67; 95% CI: 0.52–0.86) (no p-value specified)2 |
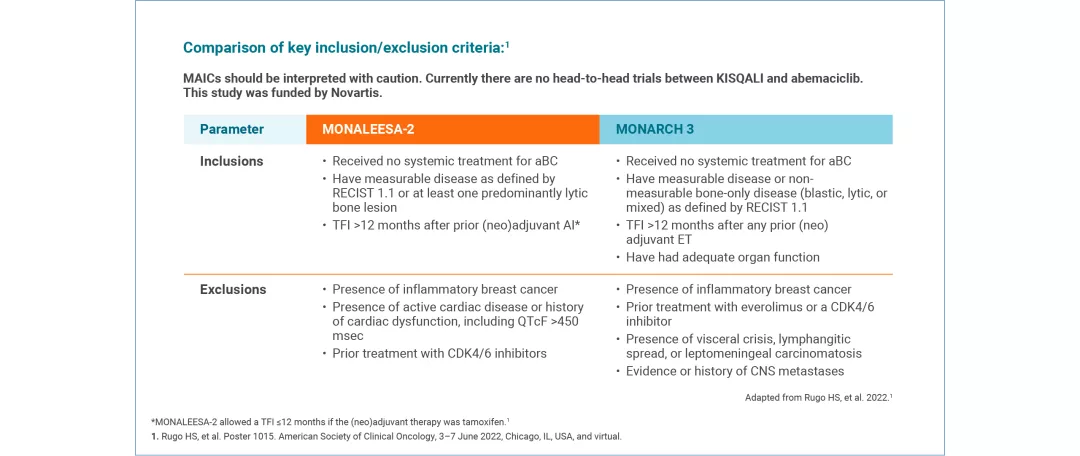
KISQALI + AI (INDIRECT COMPARISON: MONALEESA-2 VS MONARCH 3) This MAIC study was funded by Novartis Pharma AG. | A matching-adjusted indirect comparison (MAIC) investigated symptom-related QoL in 1L KISQALI + AI vs abemaciclib + AI9 No head-to-head data comparing KISQALI and abemaciclib are available. Results must be interpreted with caution. |
Results are from individual studies. They are presented together for illustrative purposes only and should not be directly compared.
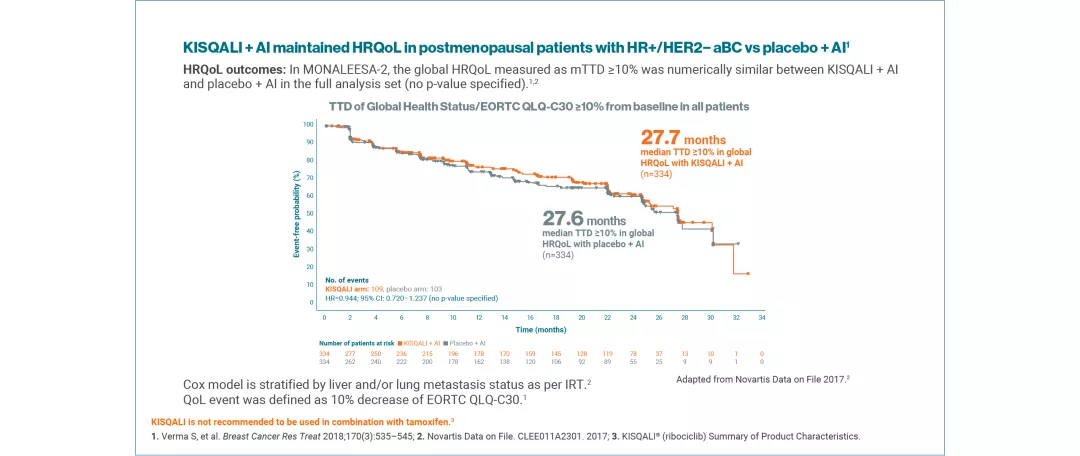
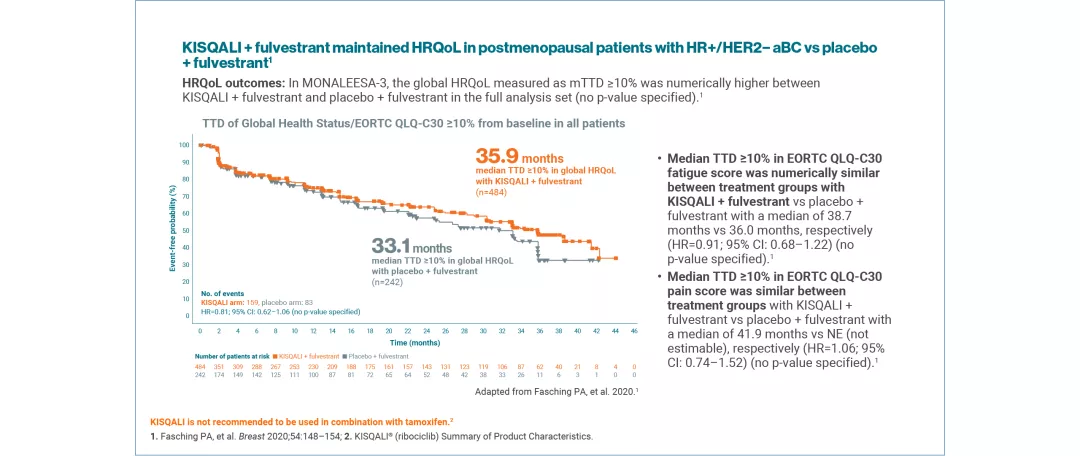
QoL was assessed using the EORTC QLQ-C30 questionnaire. Time to definitive deterioration 10% (TTD; defined as ≥10% worsening of the scales score relative to baseline, with no later improvement above this threshold observed during the treatment period, or death due to any cause) in the global health status/QoL score was investigated.4,5
KISQALI is not recommended to be used in combination with tamoxifen.1
Explore the efficacy outcomes in patients treated with KISQALI + ET*
Learn more about ESMO-MCBS and KISQALI
Study designs
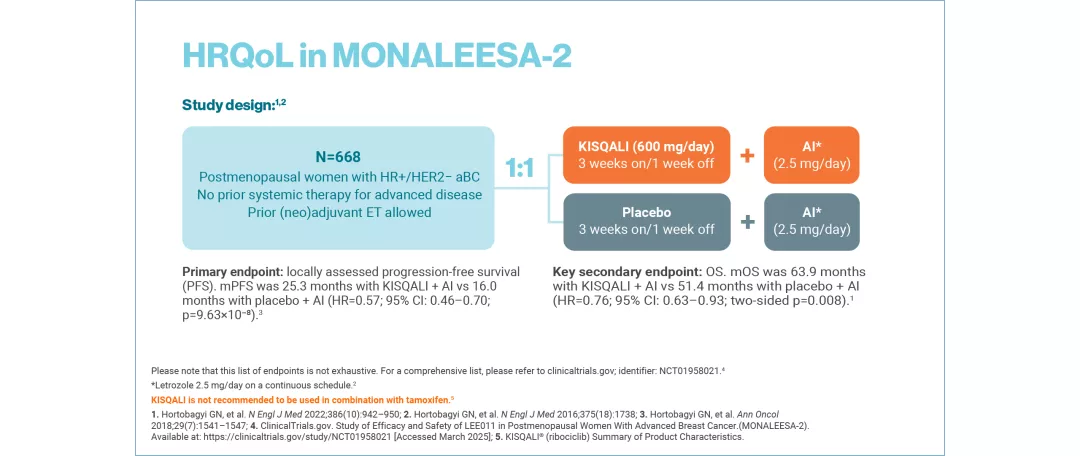
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, Phase III trial in postmenopausal women with HR+/HER2− aBC. As 1L in advanced disease. No prior ET for aBC and no previous systemic chemotherapy for advanced disease. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + AI (2.5 mg continuous). The primary endpoint was locally assessed PFS and the key secondary endpoint was OS. Other secondary endpoints included the overall response rate (complete or partial response), the clinical benefit rate (overall response plus stable disease lasting 24 weeks or more), safety and QoL assessments.11
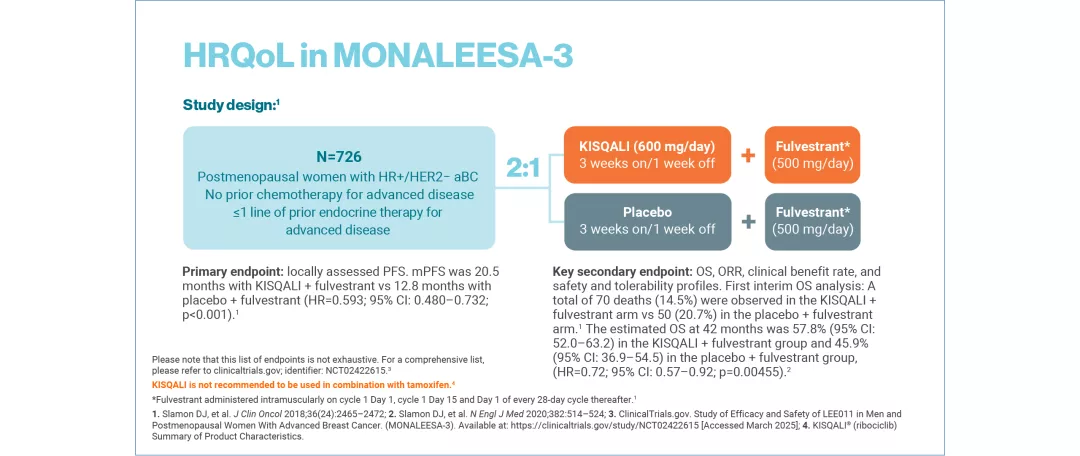
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, Phase III trial. As 1L and 2L in advanced disease plus those with early relapse in postmenopausal women with HR+/HER2– aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. The primary endpoint was locally assessed PFS. Secondary endpoints included OS, ORR, clinical benefit rate, and safety and tolerability. No prior endocrine therapy or after disease progression on 1L ET for aBC.12
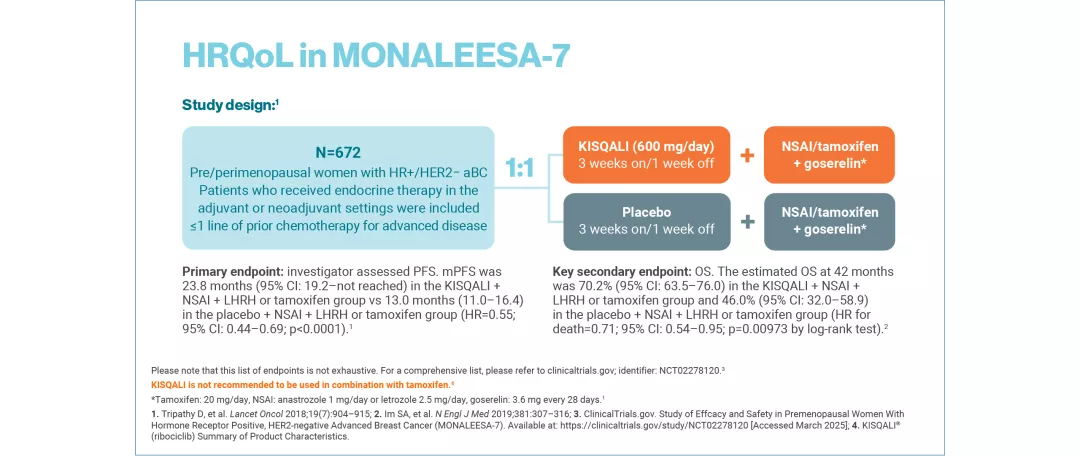
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, Phase III trial in pre/perimenopausal women with HR+/HER2− aBC. As 1L in advanced disease and in patients who received 1 or fewer lines of chemotherapy for aBC. KISQALI 600 mg or placebo orally once daily (3 weeks on/1 week off) + NSAI (letrozole 2.5 mg or anastrozole 1 mg) or tamoxifen 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on Day 1 of every cycle). The primary endpoint was investigator-assessed PFS. The key secondary endpoint was OS, defined as the time from randomisation to death from any cause. The other secondary endpoints included: proportion of patients who achieved an objective response, clinical benefit, time to response, duration of response, time to definitive deterioration of ECOG performance status from baseline, time to 10% deterioration for EORTC QLQ-C30 and safety.13
KISQALI is not recommended to be used in combination with tamoxifen.1
*ET is defined as AI or fulvestrant and LHRH.13
1L, first-line; 2L, second-line; aBC, advanced breast cancer; AI, aromatase inhibitor; ALT, alanine aminotransferase; CDK4/6; cyclin-dependent kinases 4 and 6; CI, confidence interval; CNS, central nervous system; ECOG Eastern Cooperative Oncology Group; EORTC, European Organisation for NResearch and Treatment of Cancer; ESMO, European Society of Clinical Oncology; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; HRQoL, health-related quality of life; ITT, intention-to-treat; LHRH, lutenising hormone-releasing hormone; MAIC, matching-adjusted indirect comparison; MCBS, magnitude of clinical benefit scale; mOS, median overall survival; mPFS, median progression-free survival; NSAI, non-steroidal aromatase inhibitor; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; QLQ-BR23, quality of life questionnaire for breast cancer; QLQ-C30, quality of life questionnaire for cancer patients; QoL, quality of life; QTcF, corrected QT interval by Fredericia’s formula; RECIST, Response Evaluation Criteria in Solid Tumors; SmPC, summary of product characteristics; TFI, treatment-free interval; TTD, time to deterioration; TTSD, time to sustained deterioration.
References
KISQALI® (ribociclib) Summary of Product Characteristics.
Harbeck N, et al. Ther Adv Med Oncol 2020;12:1–8.
Verma S, et al. Breast Cancer Res Treat 2018;170(3):535–545.
Fasching PA, et al. Breast 2020;54:148–154.
Beck JT, et al. Cancer Res. 2019;79 (4_Supplement):P6-18-14.
Paraskevi T. Oncol Rev 2012;6(1):e2.
Musoro JZ, et al. Eur J Cancer 2023;188:171–182.
Novartis Data on File. CLEE011A2301. 2017.
Rugo HS, et al. Poster 1015. American Society of Clinical Oncology, 3–7 June 2022.
European Society for Medical Oncology. Ribociclib. Available at: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-scorecards?mcbs_score_cards_form%5BsearchText%5D=ribociclib [Accessed April 2025].
Hortobagyi GN, et al. N Engl J Med 2016;375(18):1738–1748.
Slamon DJ, et al. J Clin Oncol 2018;36(24):2465–2472.
Tripathy D, et al. Lancet Oncol 2018;19:904–915.
UK | April 2025 | 442204
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.