This link will take you to the electronic medicines compendium (emc) website, which is a non-Novartis website.


Results from a prespecified 5-year analysis of KISQALI® (ribociclib) + NSAI vs NSAI in HR+/HER2− eBC from the pivotal NATALEE trial1,2
Indications:1
KISQALI in combination with an aromatase inhibitor (AI), is indicated for the adjuvant treatment of patients with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative early breast cancer (eBC) at high risk of recurrence (see section 5.1 of the SmPC for eligibility criteria)
In pre/perimenopausal women, or in men, the AI should be combined with a luteinising hormone-releasing hormone (LHRH) agonist
KISQALI is not recommended to be used in combination with tamoxifen.
For more information on the safety profile of KISQALI in eBC, click here.
Please consult your local Summary of Product Characteristics for the full KISQALI safety and tolerability profile. .
The NATALEE trial was a multicentre, randomised, open-label Phase III clinical trial of KISQALI + NSAI vs NSAI alone in the adjuvant treatment of HR+/HER2– eBC (N=5101). Patients received KISQALI 400 mg/d (3 weeks on/1 week off) + NSAI for 3 years while NSAI continued ≥5 years. Any ET was permitted for ≤1 year prior to randomisation. Men and premenopausal women also received goserelin.1–3
The primary endpoint of the NATALEE trial* was met at the primary analysis (11 January 2023 cut-off). A statistically significant improvement in iDFS (HR=0.748; 95% CI: 0.618–0.906; 1-sided stratified log-rank test p=0.0014) was demonstrated in patients receiving KISQALI + NSAI vs NSAI alone.1,4,5
At 5 years, invasive disease-free survival (iDFS) benefit was sustained with KISQALI + NSAI vs NSAI alone2
iDFS (ITT population)2,3
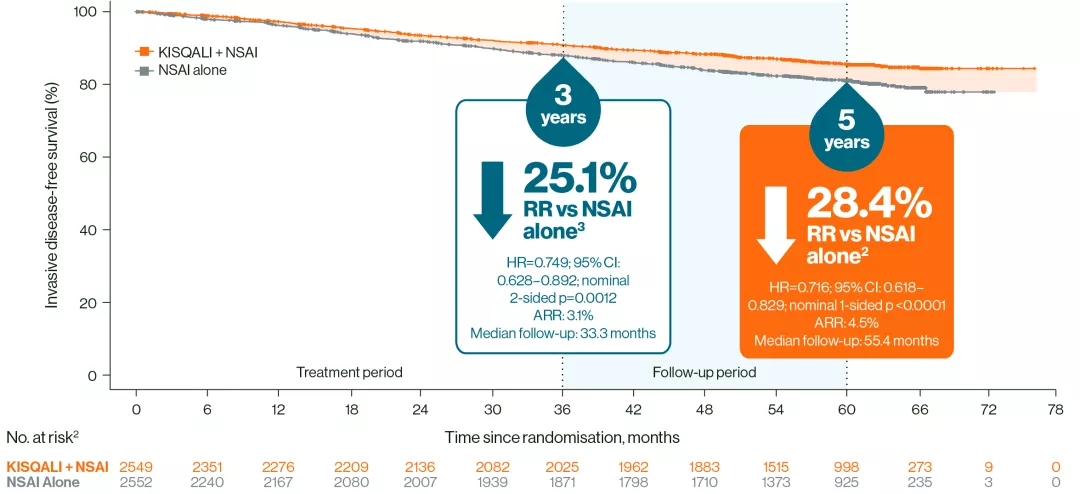
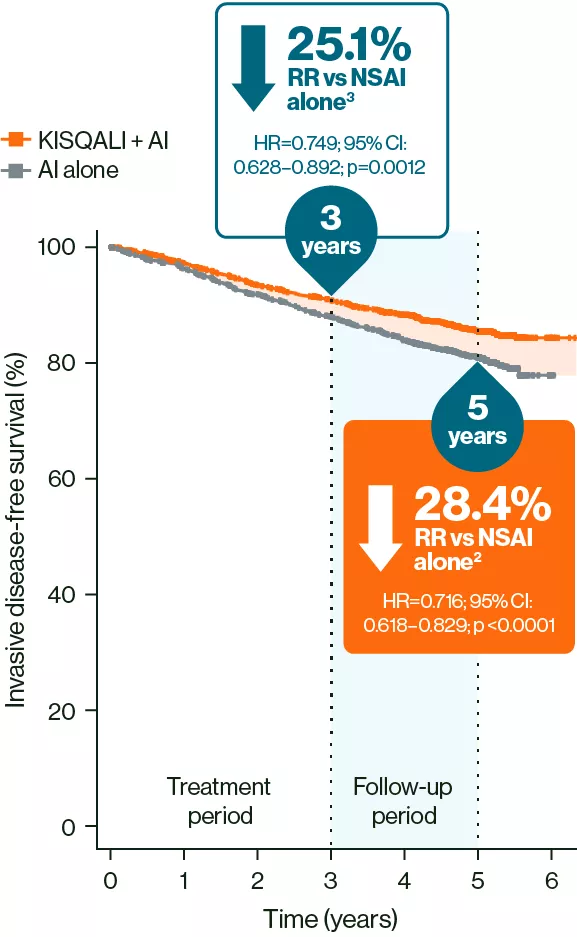
Adapted from Crown JP, et al. 20252 and Hortobagyi GN, et al. 2025.3
These results should not be regarded as statistically significant; nominal p values are shown.
At 5 years, a sustained reduction in distant disease-free survival (DDFS) events was observed with KISQALI + NSAI vs NSAI alone2
DDFS2,3
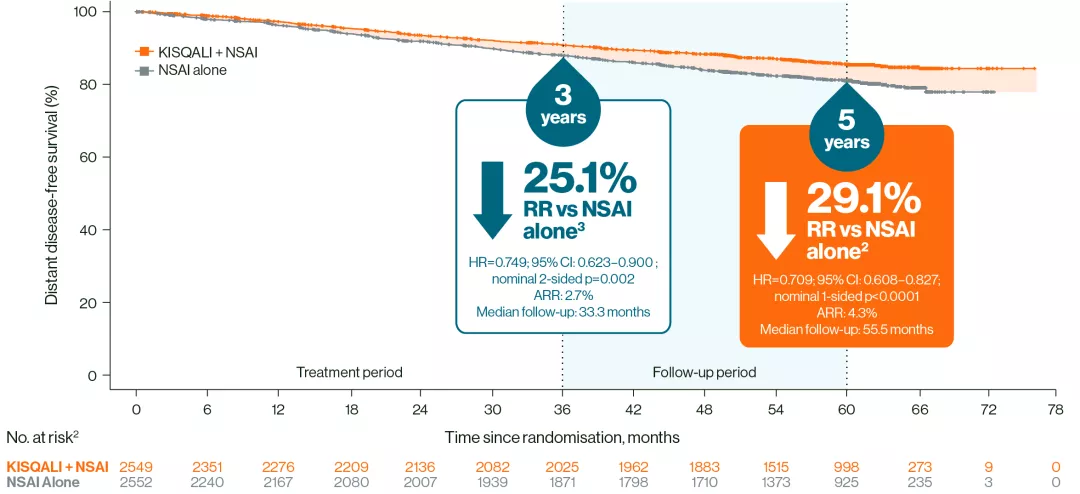
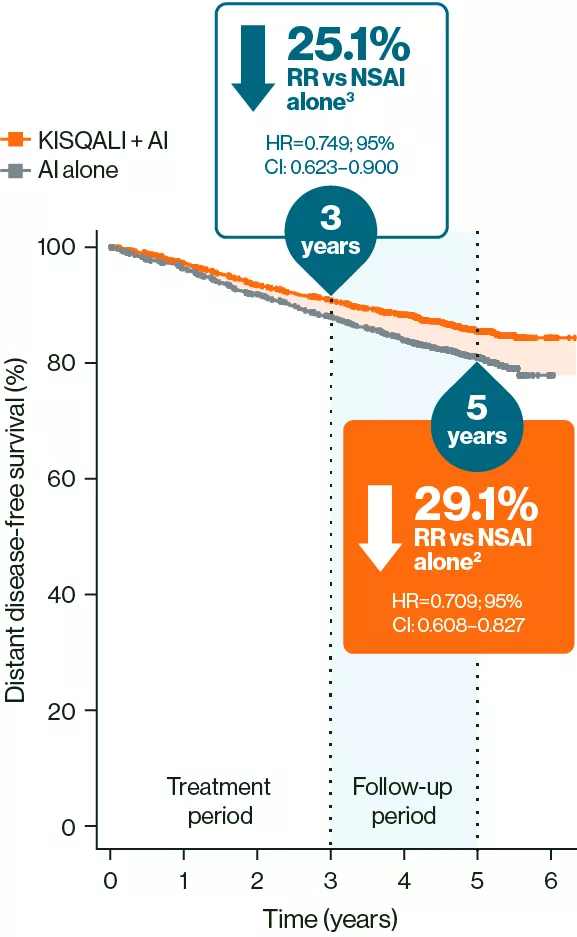
Adapted from Crown JP, et al. 20252 and Hortobagyi GN, et al. 2025.3
These results should not be regarded as statistically significant; nominal p values are shown.
As OS data matures, a numerical trend favouring KISQALI + NSAI vs NSAI alone continued to be observed2
| KISQALI + NSAI (n=2549) | NSAI alone (n=2552) |
5-year OS events, n | 138 | 162 |
HR (95% CI) | 0.800 (0.637–1.003) | |
Nominal one-sided p | 0.026 | |
Adapted from Crown JP, et al. 2025.2
These results should not be regarded as statistically significant; nominal p values are shown.
KISQALI + NSAI was observed to reduce iDFS risk up to 5 years in a broad range of patients vs NSAI alone, including high-risk N0†2
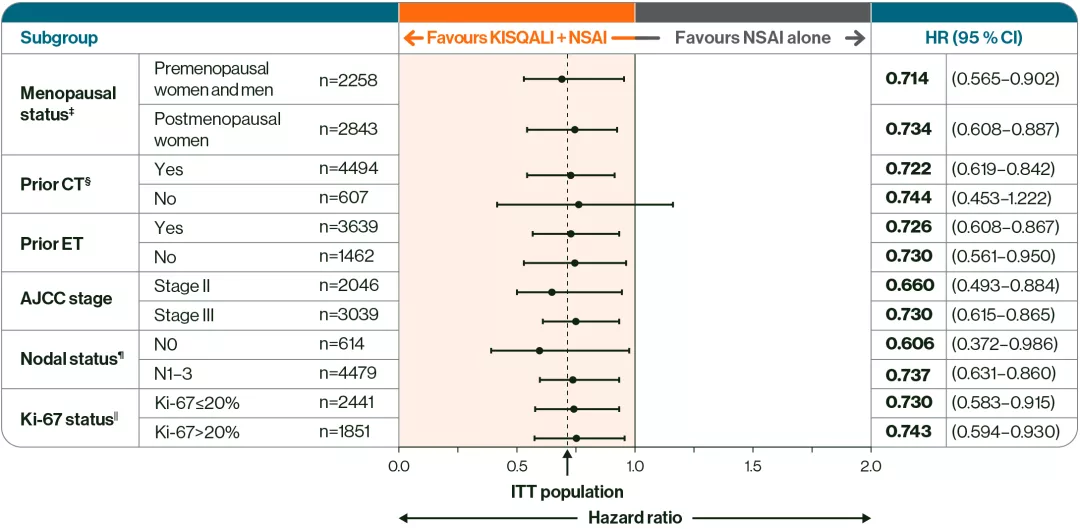
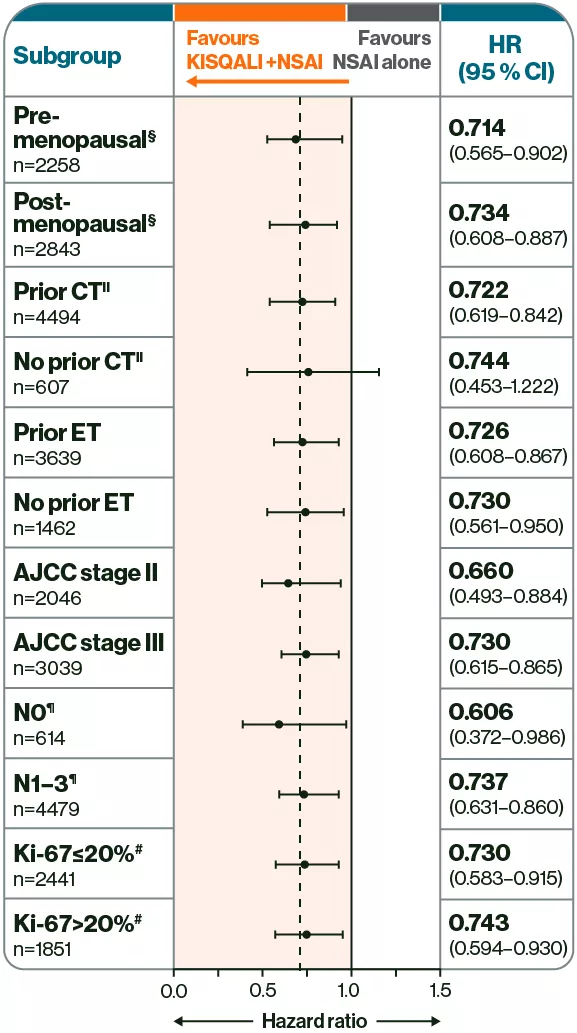
Adapted from Crown JP, et al. 2025.2
Prescribe KISQALI + AI for your N+ and high-risk N0 patients with HR+/HER2– eBC who have received adjuvant ET for ≤1 year1
KISQALI is not recommended to be used in combination with tamoxifen.1

Sustained iDFS benefit at 5 years vs NSAI alone2

Reduced iDFS risk across a broad range of N+ and high-risk N0† patients vs NSAI alone2

A generally manageable and consistent safety profile1–3
Summary of safety profile for KISQALI
The most common adverse drug reactions (reported at a frequency ≥20%) in the dataset for which the frequency for KISQALI + NSAI exceeds the frequency for NSAI alone were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests. The most common grade 3/4 adverse drug reactions (reported at a frequency of ≥2%) in the dataset for which the frequency for KISQALI + NSAI exceeds the frequency for NSAI alone were neutropenia, abnormal liver function tests and leukopenia.1
Dose reduction and permanent discontinuation due to adverse events, occurred in 22.8% and 19.5% of patients receiving KISQALI + AI, respectively in the NATALEE trial.3
For more information, see the Summary of Product Characteristics.
After 3 years of follow-up, KISQALI 400 mg had a generally manageable and well characterised safety profile3
In the NATALEE trial, the most common adverse events of any grade were neutropenia (62.5% with KISQALI + NSAI versus 4.6% with NSAI alone), arthralgia (37.3% versus 43.3%, respectively), and nausea (23.3% versus 7.8%, respectively). The most common grade ≥3 event was neutropenia (44.3% with KISQALI + NSAI versus 0.9% with NSAI alone)3
No new safety signals were identified during the 5-year analysis. The proportion of patients who developed secondary primary malignancies was similar between the two arms.** With 12.9 months of additional follow-up, five new deaths due to adverse events occurred since the prior 4-year analysis‡‡2
Learn more about the safety profile of KISQALI in eBC
*iDFS per STEEP criteria.5
†N0 patients with with either grade 3 histology, grade 2 histology with Ki-67 ≥20%, or grade 2 histology with high genomic risk.2
‡Included men and premenopausal women.2
§Includes neoadjuvant and adjuvant chemotherapy.2
¶Nodal status classification according to AJCC staging at the most advanced stage derived per surgical specimen or at diagnosis.2
‖From archival tumour tissue.2
**KISQALI + NSAI: 67 patients (2.7%); NSAI alone: 74 (3.0%).2
‡‡These deaths were not considered related to study treatment and occurred in three patients in the KISQALI + NSAI group (brain haemorrhage, myocardial infarction and gastric adenocarcinoma) and two in the NSAI-alone group (rectal adenocarcinoma andaortic aneurysm rupture).2
ABC, advanced breast cancer; AE, adverse event; AI, aromatase inhibitor; AJCC, American Joint Committee on Cancer; ARR, absolute risk reduction; CI, confidence interval; DDFS, distant disease-free survival; eBC, early breast cancer; ESMO, European Society for Medical Oncology; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2-negative; HR, hazard ratio; HR+, hormone receptor-positive; iDFS, invasive disease-free survival; ITT; intention to treat; LHRH, luteinising hormone-releasing hormone; N+, node positive; N0, node negative; NSAI, non-steroidal aromatase inhibitor; OS, overall survival; RR, risk reduction; SmPC, summary of product characteristics.
References
KISQALI (ribociclib) Summary of Product Characteristics.
Crown JP, et al. ESMO Open 2025;doi:10.1016/j.esmoop.2025.105858.
Hortobagyi GN, et al. Ann Oncol 2025;36(2):149–157.
Slamon DJ, et al. J Clin Oncol 2023;41(suppl 17):LBA500.
Slamon DJ, et al. Ther Adv Med Oncol 2023;15:1–16.
UK | November 2025 | FA-11537997
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.

