
Protection
Understanding Valsartan’s Protective Benefits
Beyond its proven efficacy in lowering blood pressure, Valsartan offers broad cardiovascular protection1 supported by clinical evidence. This section highlights key trials demonstrating its role in reducing the risk of different cardiovascular diseases, and supporting patients with high cardiovascular risk.
Explore how Valsartan extends its value beyond BP control to comprehensive heart health.
Valsartan benefits are beyond BP control
44,476 patients enrolled
Image

| Image
|
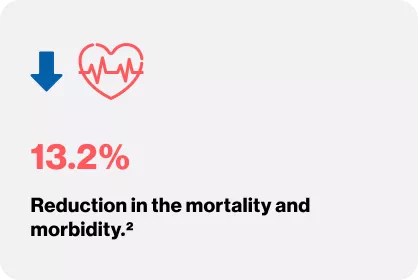
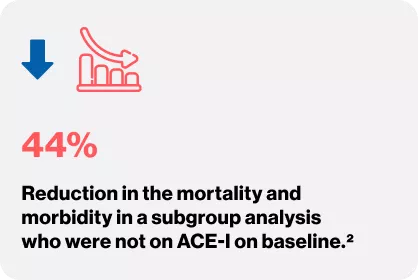
VALHEFT TRIAL
SVG
| 5,010 patients |

VALUE TRIAL
SVG
| 15,245 patients |
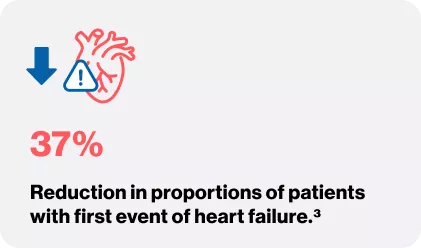

VALIANT TRIAL
SVG
| 14,703 patients |
VALUE TRIAL
SVG
| 15,245 patients |

NAVIGATOR TRIAL
SVG
| 9,518 patients |

ACE-I: Angiotensin converting enzyme inhibitor
References
Egyptian drug authority Tareg approved leaflet; approval date: 26/10/2020.
Cohn JN, Tognoni G. A randomized trial of the angiotensin-receptor blocker valsartan in chronic heartfailure. New England Journal of Medicine. 2001 Dec 6;345(23):1667-75.
Julius S, Weber MA, Kjeldsen SE, McInnes GT, Zanchetti A, Brunner HR, Laragh J, Schork MA, Hua TA,Amerena J, Balazovjech I. The valsartan antihypertensive long-term use evaluation (VALUE) trial: outcomes in patients receiving monotherapy. Hypertension. 2006 Sep 1;48(3):385-91.
Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT,Mitchell L, Plat F. Outcomes in hypertensive patients at high cardiovascular risk treated with regimensbased on valsartan or amlodipine: the VALUE randomised trial. The Lancet. 2004 Jun 19;363(9426):2022-31.
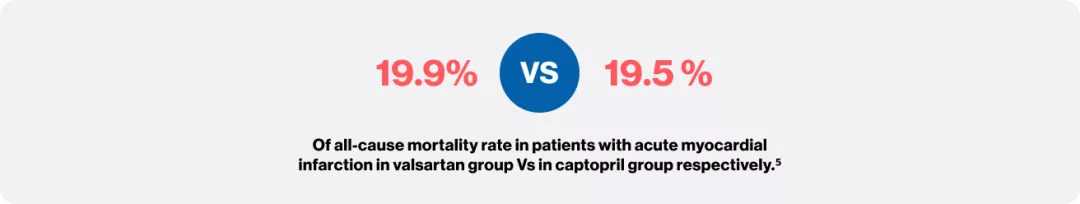
Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Køber L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. New England Journal of Medicine. 2003 Nov 13;349(20):1893-906.
NAVIGATOR Study Group. Effect of valsartan on the incidence of diabetes and cardiovascular events. New England Journal of Medicine. 2010 Apr 22;362(16):1477-90
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
