Renal Impairment: Exforge HCT is contraindicated in patients with severe renal impairment and patients undergoing dialysis
Hepatic Impairment: Exforge HCT is contraindicated in patients with hepatic impairment, biliary cirrhosis, or cholestasis
Egyptian drug authority Exforge-HCT Leaflet; approval date: 4/5/2025.\
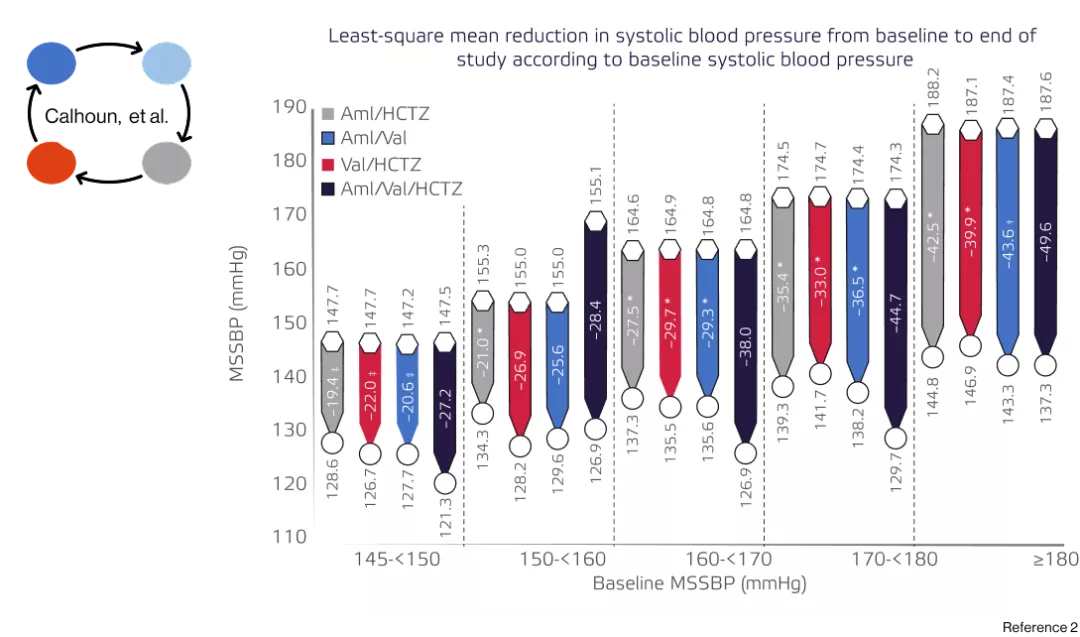
Calhoun DA, Crikelair NA, Yen J, Glazer RD.Amlodipine/valsartan/hydrochlorothiazide triple combination therapy in moderate/severe hypertension: secondary analyses evaluating efficacy and safety. Advances in therapy. 2009 Nov;26:1012-23
Mancia Chairperson G, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Eae A, Azizi M, Benetos A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). Journal of hypertension. 2023;41(12):1874-2071.
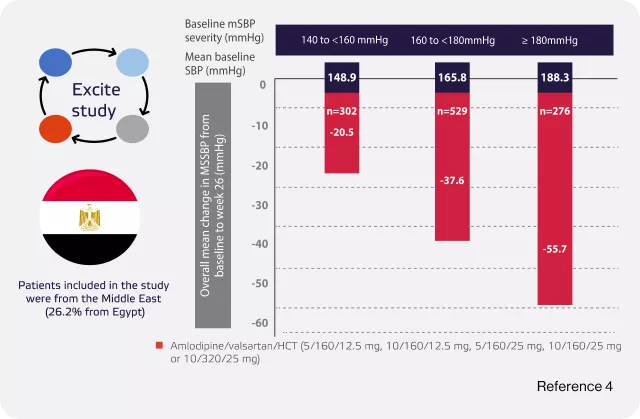
Sison J, Assaad-Khalil SH, Najem R, Kitchlew AR, Cho B, Ueng KC, Shete A, Knap D. Real-world clinical experience of amlodipine/valsartan and amlodipine/valsartan/hydrochlorothiazide in hypertension: the EXCITE study. Current Medical Research and Opinion. 2014 Oct 1;30(10):1937-45
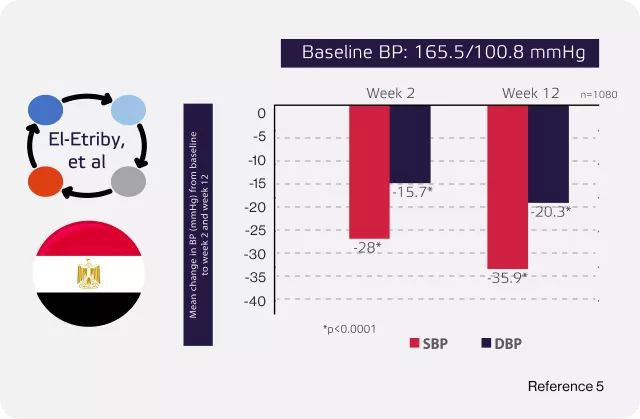
El-Etriby AM, Rakha S. Efficacy and safety of amlodipine/valsartan/hydrochlorothiazide single pill combination in Egyptian patients with hypertension uncontrolled on any dual therapy: an observational study. Current Medical Research and Opinion. 2020 Apr 2;36(4):537-44.
Exforge HCT®
Exforge HCT is a combination medication used to treat high blood pressure (hypertension) in adults. It combines three active ingredients: amlodipine, a calcium channel blocker; valsartan, an angiotensin II receptor blocker (ARB); and hydrochlorothiazide, a thiazide diuretic.1
This combination works synergistically to lower blood pressure more effectively than any of the components alone.1
For patients uncontrolled over dual therapy, average of 50mmhg blood pressure reduction based on calhoun trial is provided by exfoge HCT2

Calhoun trial : There was a significant decrease in mean systolic blood pressures (MSSBPs) across different levels of severities, with a more pronounced reduction observed in cases where the baseline MSSBP was higher.2
Egyptian Studies for Exforge HCT
A significant change in BP was observed after 12 weeks from SBP 165.5 mmHg at baseline to 129.7 mmHg.5
Image

| Dosage and administration1The recommended dose of Exforge HCT is one tablet per day. |


Image

| Clinical considerations1
|
References
Exforge HCT® API
Exforge HCT® API
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |