
Renal Impairment: No dose adjustment is required for patients with mild to moderate renal impairment.
Hepatic Impairment: Exforge is contraindicated in patients with severe hepatic impairment. Caution should be exercised when administering Exforge to patients with hepatic impairment or biliary obstructive disorders
Geriatric Patients: In elderly patients, caution is required when increasing the dosage
Summary of product characteristics. Exforge. Available at: https://www.ema.europa.eu/en/documents/product-information/exforge-epar-product-information_en.pdf; last accessed: 17/7/2025.
Allemann Y, Fraile B, Lambert M, Barbier M, Ferber P, Izzo Jr JL. Efficacy of the combination of amlodipine and valsartan in patients with hypertension uncontrolled with previous monotherapy: the Exforge in Failure after Single Therapy (EX‐FAST) study. The Journal of Clinical Hypertension.2008 Mar;10(3):185-94
Mancia Chairperson G, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Eae A, Azizi M, Benetos A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). Journal of hypertension. 2023;41(12):1874-2071.
Destro M, Luckow A, Samson M, Kandra A, Brunel P. Efficacy and safety of amlodipine/valsartan compared with amlodipine monotherapy in patients with stage 2 hypertension: a randomized, double-blind, multicenter study: the EX-EFFeCTS Study. Journal of the American Society of Hypertension. 2008 Jul 1;2(4):294-302
Egyptian drug authority Exforge approved leaflet; approval date: 4/5/2025.
Exforge®
Exforge is a combination medication used to treat high blood pressure in adults whose blood pressure is not adequately controlled on either amlodipine or valsartan monotherapy. It combines amlodipine, a calcium channel blocker, with valsartan, an angiotensin II receptor blocker (ARB). This combination works synergistically to lower blood pressure more effectively than either component alone.1
Exforge 5-10/160 mg: An effective and well-tolerated strategy for BP control in a wide range of patients with HTN not previously controlled by use of a single antihypertensive agent.2
Exforge for Adults with moderate essential hypertension and type 2 diabetes1

At Week 8, 21.9% of diabetic patients in the Exforge group had achieved an SBP <130 mm Hg compared with 5.6% in the amlodipine arm.4
Changes in MSSBP favored combination therapy at weeks 4 and week 8.4
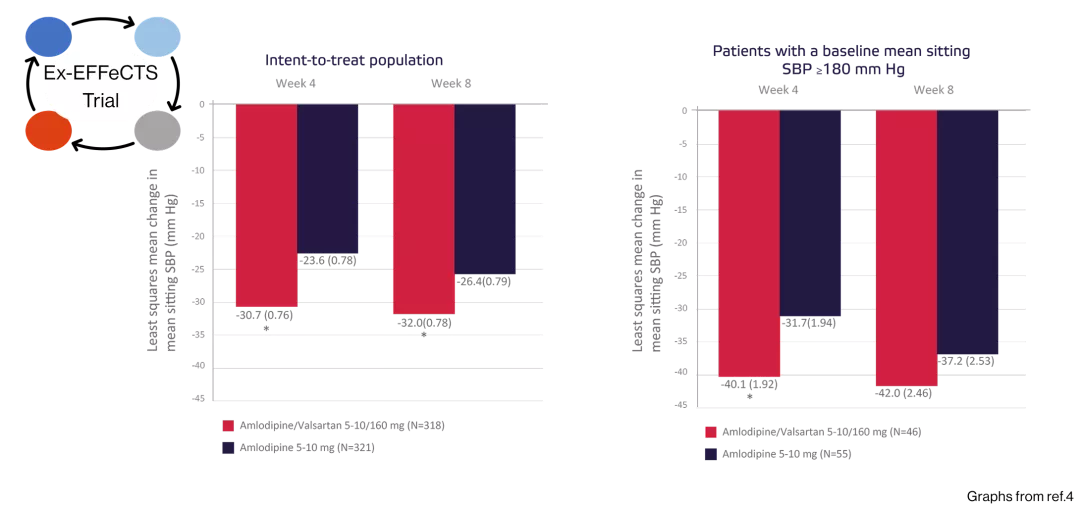
Mean change from baseline in mean sitting systolic blood pressure (SBP) according to treatment at weeks 4 and 8
MSSBP reductions at week 8 were in the range of 19.5 to 24.3 mm Hg for the 10/160 mg treatment arm2
Reference 2
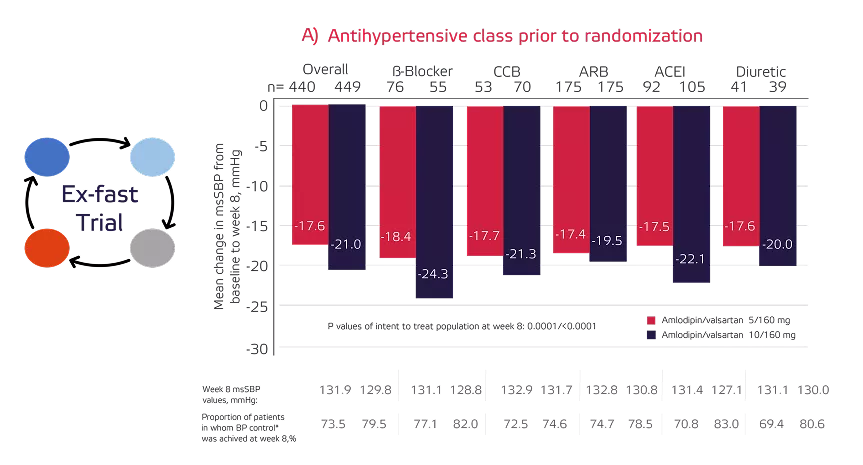
The impact of prior antihypertensive monotherapy in patients switched directly to amlodipine/valsartan on mean change in mean sitting systolic blood pressure (MSSBP) from baseline to week 8
Image

| Dosage and administration5The recommended dose of Exforge is one tablet per day. |


Image

| Clinical considerations5
|
References
Exforge® API
Exforge® API
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
