
24-hr control
ESC 2024 guidelines promote the use of out-of-office measurement (ambulatory monitoring - usually for a 24-h period) for diagnosis and ongoing management of hypertension.1
Valsartan has demonstrated consistent 24-hour efficacy, including during critical early morning hours when cardiovascular risk peaks.2
The BP control is essential
Poor control of blood pressure resulting in changes to antihypertensive drugs also affects variability. Long term variability in blood pressure measured in adults at clinic visits is associated with cardiovascular and mortality outcomes.3


Mean±SD decreases from baseline in 24-hour, daytime, and nighttime systolic blood pressure obtained on ambulatory blood pressure monitoring after 2 and 8 weeks of treatment with olmesartan and valsartan.2

Reference 2
Twenty-four-hour systolic and diastolic blood pressure at baseline and after 2 and 8 weeks of treatment with valsartan.2

Reference 2
*P<.001 vs baseline; †P<.001 vs olmesartan; ‡P<.01 vs olmesartan
Single-pill combinations (SPCs) are recommended by major hypertension guidelines, including the ESC1 and ESH7, for patients requiring multi-drug therapy.
SPCs simplify treatment regimens, improve adherence, and accelerate blood pressure control. Clinical evidence shows that patients on SPCs achieve target BP faster and with greater consistency compared to multi-pill regimens.1

Reduce pill burden1

Improve adherence1
BP-lowering treatment of resistant hypertension1
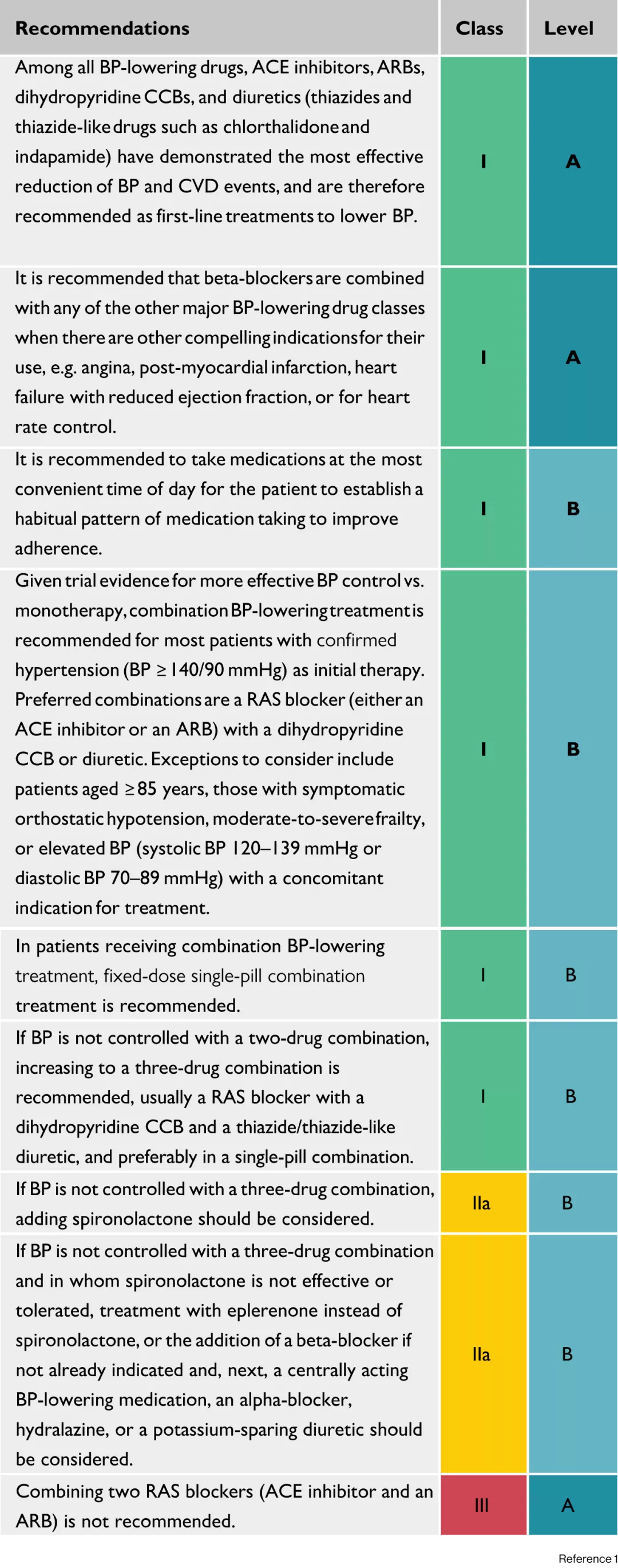
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CVD, cardiovascular disease; RAS, renin- angiotensin system.
References
McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, Christodorescu RM, Daskalopoulou SS, Ferro CJ, Gerdts E, Hanssen H. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. 2024 Aug 30;45(38):3912-4018.
Destro M, Scabrosetti R, Vanasia A, Mugellini A. Comparative efficacy of valsartan and olmesartan in mild-to-moderate hypertension: results of 24-hour ambulatory blood pressure monitoring. Advances in therapy. 2005 Jan;22:32-43.
Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ. Blood pressure variability and cardiovascular disease: systematic review and meta-analysis. bmj. 2016 Aug 9;354.
Egyptian drug authority Co-Tareg approved leaflet; approval date: 8/5/2025.
Egyptian drug authority Exforge approved Leaflet; approval date: 4/5/2025.
Egyptian drug authority Exforge-HCT Leaflet; approval date: 4/5/2025.
Mancia Chairperson G, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Eae A, Azizi M, Benetos A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). Journal of hypertension. 2023;41(12):1874-2071.
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
