
Co-Tareg®
CoTareg is a combination medication used as an initial therapy for patients with hypertension who are likely to need multiple drugs to achieve their blood pressure goals. It combines valsartan, an angiotensin II receptor blocker (ARB), with hydrochlorothiazide, a thiazide diuretic.1
This combination works synergistically to lower blood pressure more effectively than either component alone.2
Co-Tareg for newly diagnosed Moderate hypertension1

Co-tareg 160/12.5: greater sSBP and sDBP reductions of -32.1 mmHg and -16.9 mmHg were achieved in treatment naïve patients.4
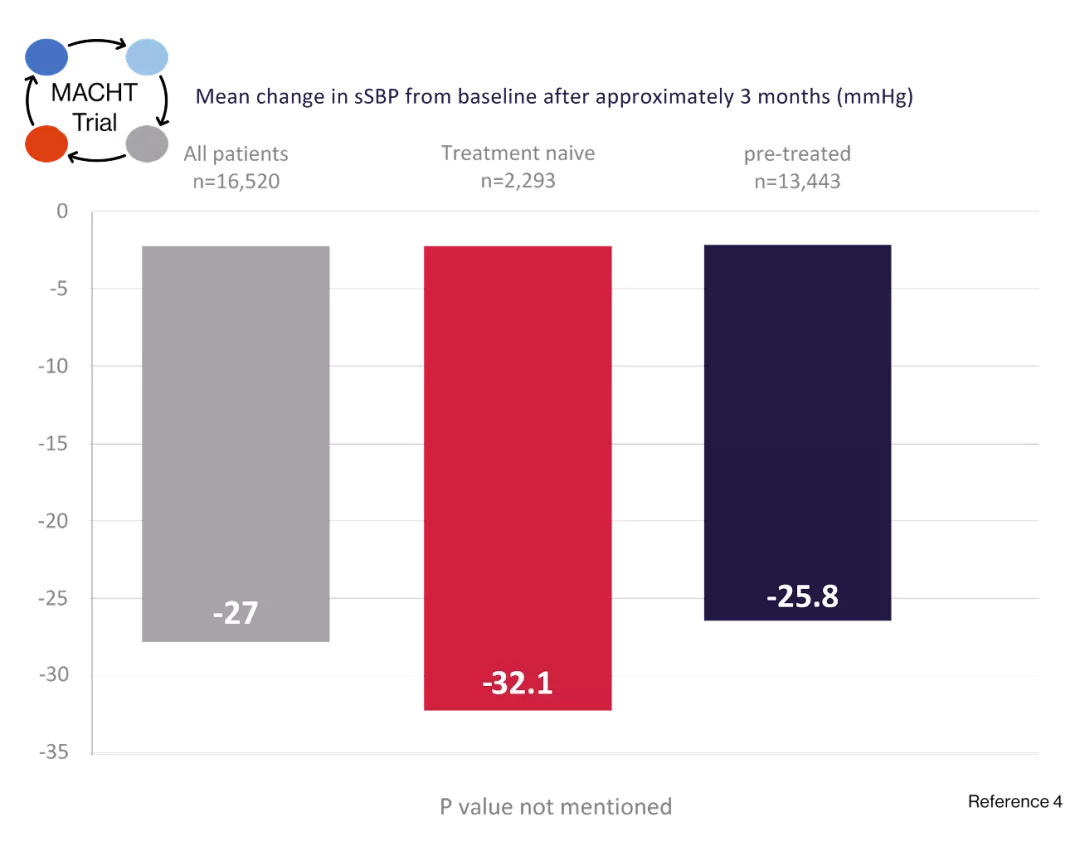
Study design4
17,242 patients were enrolled in an uncontrolled, multicenter observational study Regimen: Combined valsartan 160 mg and HCTZ 12.5 mg was prescribed according to the routine clinical practice of the physician in accordance with the Summary of Product Characteristics.
Objective4
To evaluate the effectiveness of this combination in achieving target BP levels
Image

| Dosage and administration1The recommended starting dose of CoTareg is 160 mg of valsartan and 12.5 mg of hydrochlorothiazide once daily. The maximum antihypertensive effect is typically seen within 2 to 4 weeks. |
Image

| Special considerations1
|
CV: Cardiovascular, GFR: Glomerular filtration rate.
Co-Tareg® API
Co-Tareg® API
References
Egyptian drug authority Co-Tareg approved leaflet; approval date: 8/5/2025.
Summary of product characteristics. Co-diovan. Available at: https://www.ema.europa.eu/en/documents/referral/diovan-comp-article-30-referral-annex-i-ii-iii_en.pdf; last accessed: 2/7/2025.
Mancia Chairperson G, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Eae A, Azizi M, Benetos A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). Journal of hypertension.
Abts M, Claus V, Lataster M. Blood pressure control with valsartan and hydrochlorothiazide in clinical practice: The MACHT Observational Study. Blood Pressure. 2006 Jan 1;15(sup1):27-32
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
