
Guidelines
Valsartan is endorsed across major international hypertension guidelines, including those from the European Society of Cardiology (ESC), American Heart Association (AHA), National Institute for Health and Care Excellence (NICE), and the European Society of Hypertension (ESH).
These guidelines consistently recommend angiotensin II receptor blockers (ARBs) like Valsartan as a first-line treatment option for managing elevated blood pressure, particularly in patients with compelling indications such as diabetes, heart failure, or chronic kidney disease.
2024 ESC Guidelines for the management of elevated blood pressure and hypertension1
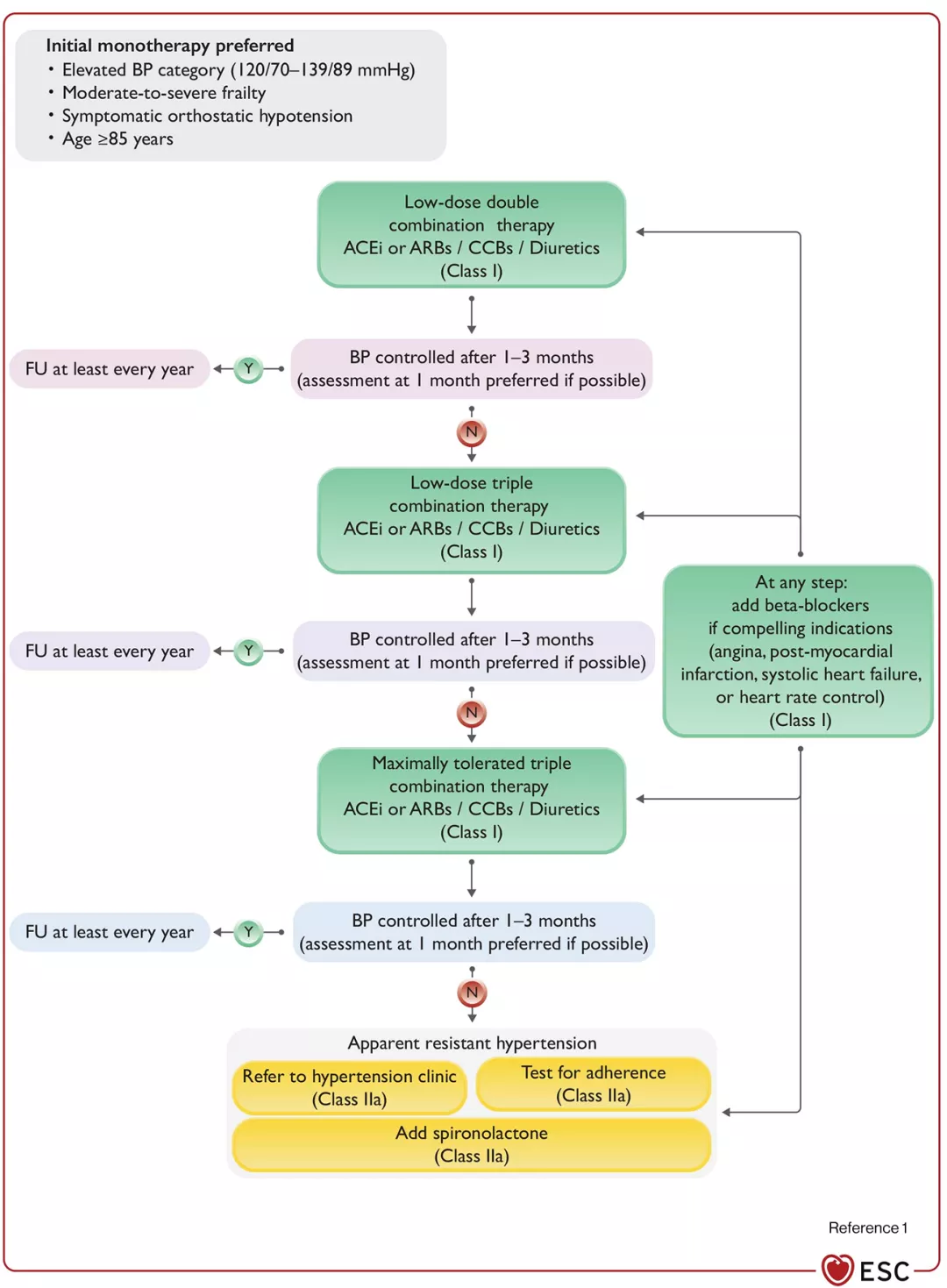
Practical algorithm for pharmacological blood pressure lowering.
Recommendations for managing hypertension in patients with chronic kidney disease1
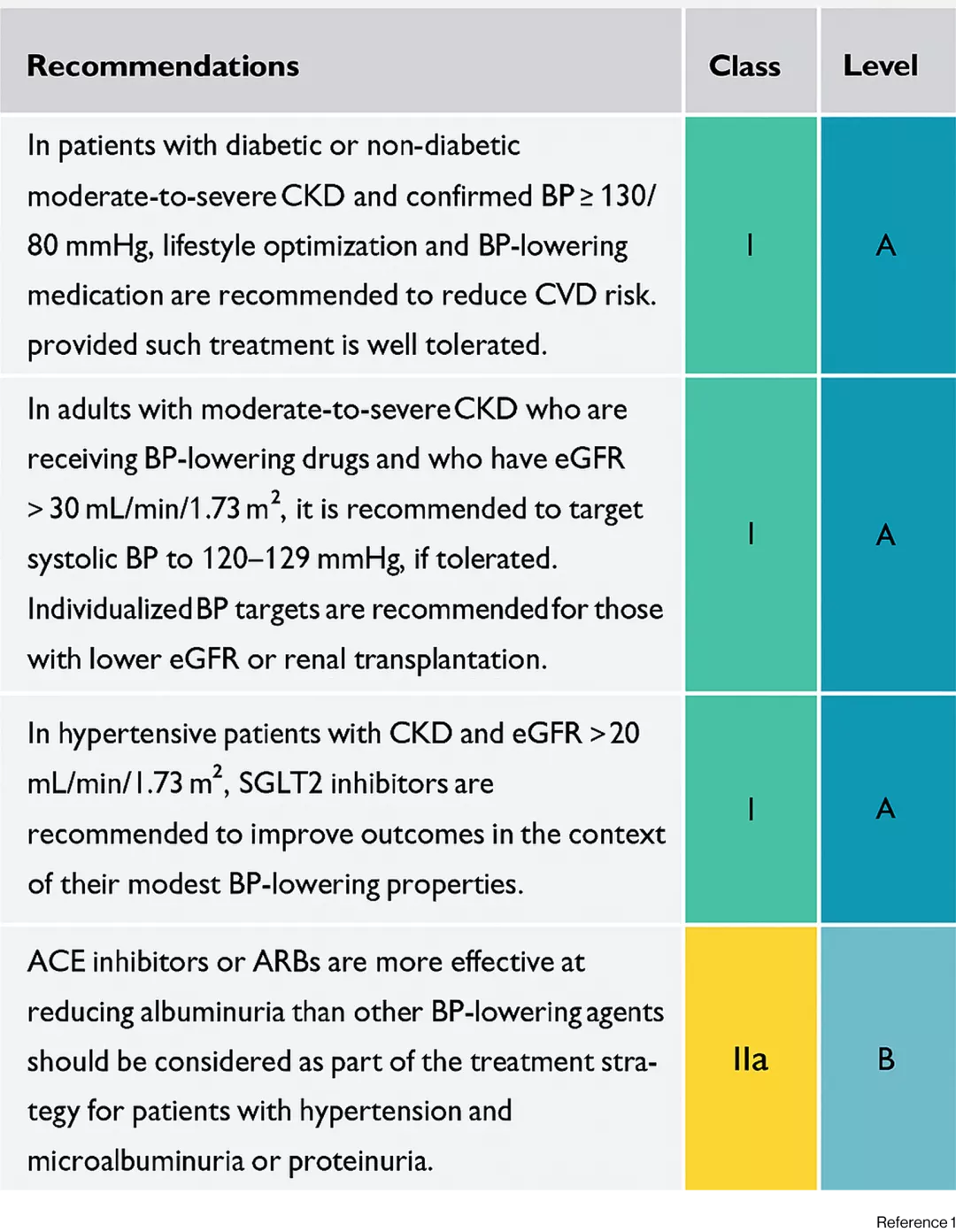
Recommendations for managing hypertension in patients with cardiac disease1
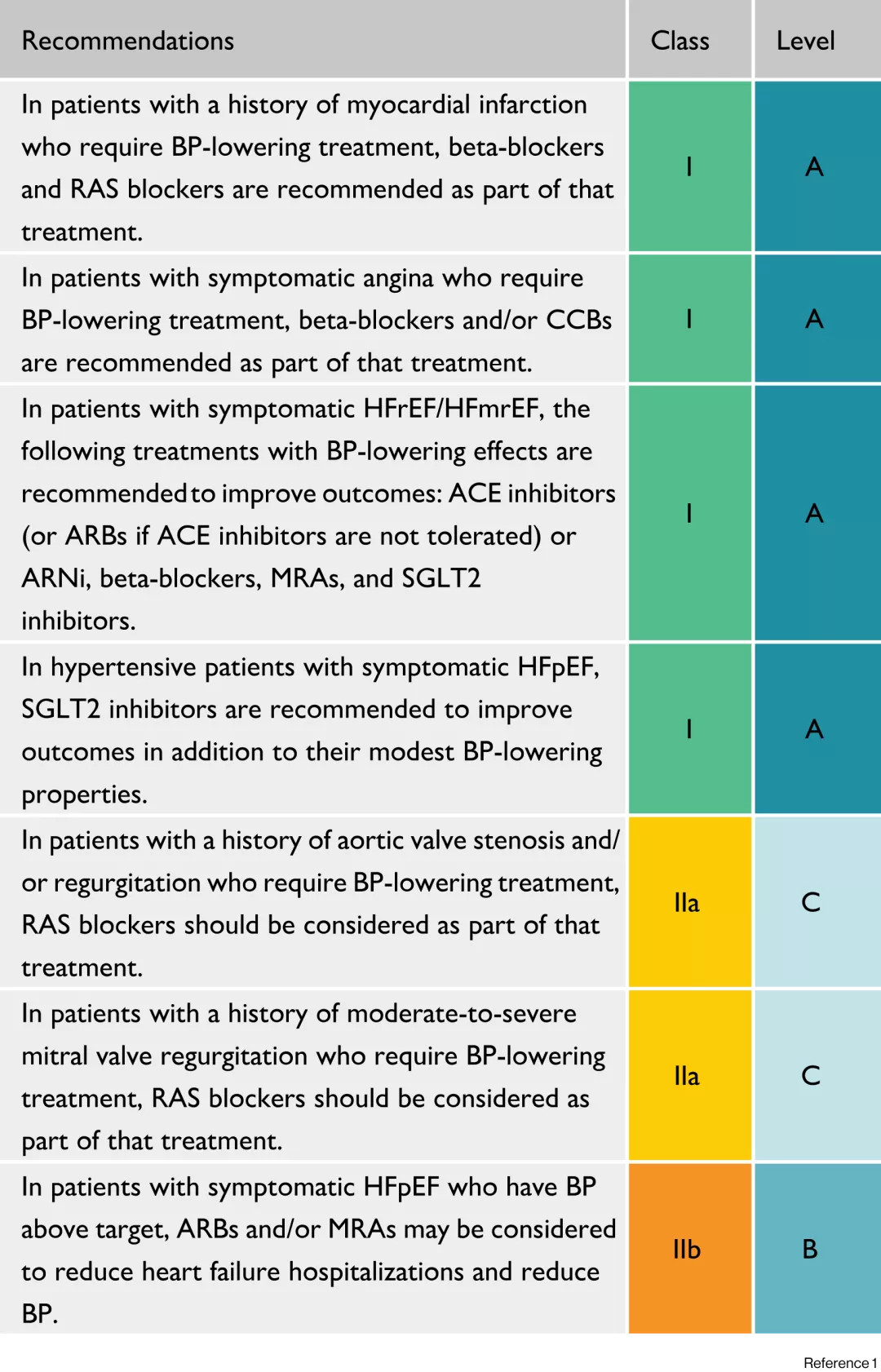
2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure2
Recommendations for Patients With Preserved LVEF (≥50%).

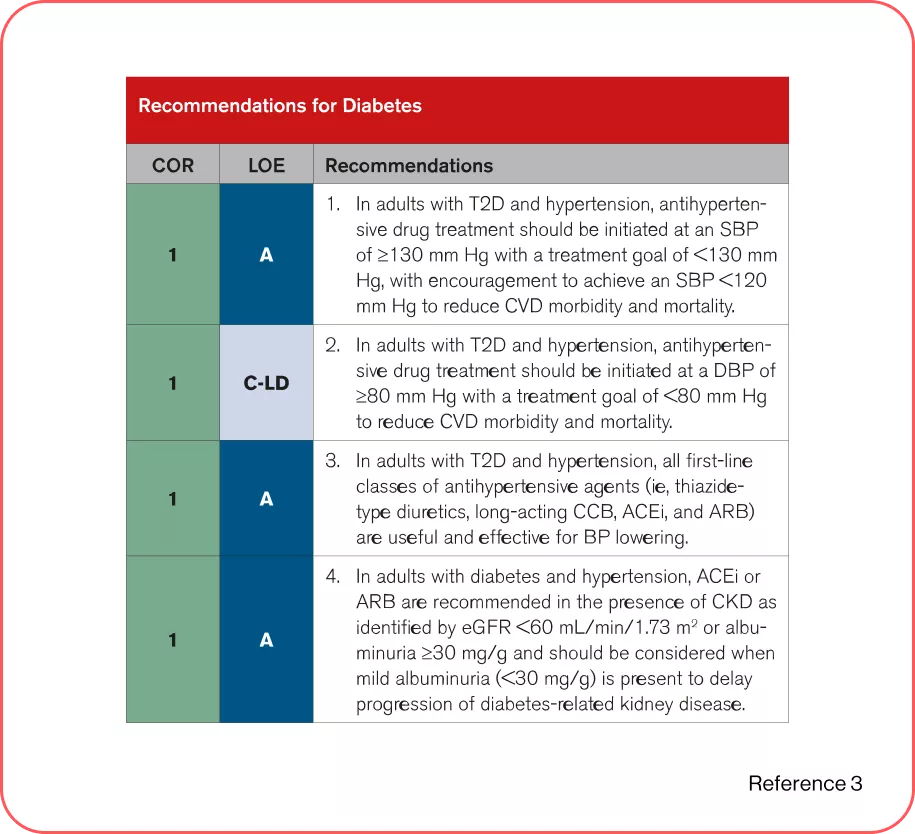
2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM Guideline for the Prevention, Detection, Evaluation and Management of High Blood Pressure in Adults3
Recommendations for Treatment of Hypertension in Patients With DM
2019 NICE guidelines: Hypertension in adults: diagnosis and management4
When choosing antihypertensive drug treatment for adults of Black African or African–Caribbean family origin, consider an angiotensin II receptor blocker (ARB), in preference to an angiotensin-converting enzyme (ACE) inhibitor. [2019] Reference 4 |
For people with cardiovascular disease: Follow the recommendations for disease-specific indications in the NICE guideline on their condition (for example, when prescribing an ACE inhibitor or an ARB for secondary prevention of myocardial infarction).
Reference 4 |
2021 NICE guidelines: Chronic kidney disease: assessment and management5
Offer an angiotensin-receptor blocker (ARB) or an angiotensin-converting enzyme (ACE) inhibitor (titrated to the highest licensed dose that the person can tolerate) to adults, children* and young people with CKD who have hypertension and an ACR over 30 mg/mmol (ACR category A3 or above). [2021] Reference 5 |
*Children from 6 years old.6
2023 ESH clinical practice guidelines for the management of arterial hypertension7
General BP-lowering strategy in patients with hypertension.

BP-lowering drugs in hypertension and heart failure

BP-lowering therapy in hypertension and HFpEF.
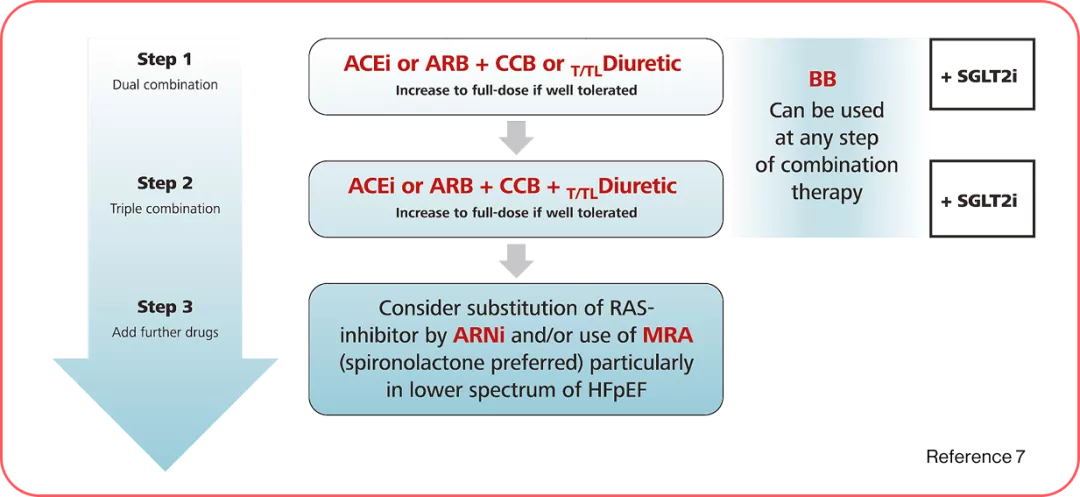
BP-lowering therapy in hypertension and CKD.

ARB: Angiotensin receptor blocker, HFrEF: Heart failure with reduced ejection fraction, HFmrEF: Heart failure with mildly reduced ejection fraction, ARNi: Angiotensin receptor–neprilysin inhibitor, MRA: Mineralocorticoid receptor antagonist, SGLT2: Sodium-glucose co-transporter 2, LVEF: Left ventricular ejection fraction, HFpEF: Heart failure with preserved ejection fraction, DM: Diabetes mellitus, CCBs: Calcium channel blocker, BB: Beta blocker
References
1.McEvoy JW, McCarthy CP, Bruno RM, Brouwers S, Canavan MD, Ceconi C, Christodorescu RM, Daskalopoulou SS, Ferro CJ, Gerdts E, Hanssen H. 2024 ESC Guidelines for the management of elevated blood pressure and hypertension. Eur Heart J. 2024 Aug 30;45(38):3912-4018.
Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC. 2022 AHA/ACC/HFSA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Joint Committee on
Clinical Practice Guidelines. Journal of the American College of Cardiology. 2022 May 3;79(17):e263-421.Jones DW, Ferdinand KC, Taler SJ, Johnson HM, Shimbo D, Abdalla M, Altieri MM, Bansal N, Bello NA, Bress AP, Carter J. 2025 AHA/ACC/AANP/AAPA/ABC/ACCP/ACPM/AGS/AMA/ASPC/NMA/PCNA/SGIM guideline for the prevention, detection, evaluation, and management
of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. JACC. 2025 Aug 14.2019 NICE guidelines. Hypertension in adults: diagnosis and management. Available at: https://www.nice.org.uk/guidance/ng136; last accessed: 2/7/2025.
2021 NICE guidelines. Chronic kidney disease: assessment and management. Available at:Overview | Chronic kidney disease: assessment and management | Guidance | NICE; lastaccessed: 2/7/2025
Egyptian drug authority Tareg approved leaflet; approval date: 26/10/2020.
Mancia Chairperson G, Brunström M, Burnier M, Grassi G, Januszewicz A, Muiesan ML, Tsioufis K, Agabiti-Rosei E, Eae A, Azizi M, Benetos A. 2023 ESH Guidelines for the management of arterial hypertension The Task Force for the management of arterial hypertension of the European Society
of Hypertension Endorsed by the European Renal Association (ERA) and the International Society of Hypertension (ISH). Journal of hypertension. 2023;41(12):1874-2071.
Approved by Egyptian Drug Authority: HF0082OA4758/102025. Invalidation date: 14/10/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at: www.edaegypt.gov.eg.
Image

|
HF0082OA4758/102025 14/10/2027 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
