Scanning for prostate-specific membrane antigen (PSMA)
Early diagnosis of prostate cancer is strongly linked to survival. Therefore, identifying reliable disease-specific biomarkers is crucial to help improve patient survival.1,2
What is PSMA?
PSMA is a biomarker used in the diagnosis of prostate cancer. It is naturally found in the prostate gland and other healthy tissues. However, its expression is increased in prostate cancer lesions, making it a valuable imaging marker.2
High levels of PSMA have been directly linked to more aggressive prostate cancer:2
5-year recurrence-free survival rates based on PSMA levels for patients on pre-operative biopsy*2
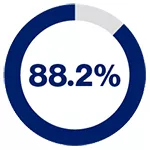
in patients exhibiting no PSMA presence
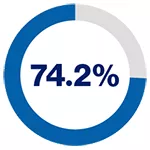
in patients exhibiting low PSMA presence
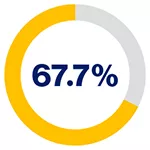
in patients exhibiting medium PSMA presence
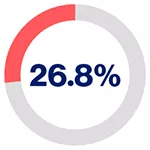
in patients exhibiting high PSMA presence
PSMA is highly expressed in ~80% of men with prostate cancer3
Prostate cancer marker
PSMA is highly expressed on prostate epithelial cells4
Upregulated in prostate cancer
PSMA is strongly upregulated in prostate cancer and with androgen deprivation4–6
Correlation with disease severity
The degree of PSMA expression positively correlates with tumour stage and early recurrence5,7
Relationship to advanced disease
The expression of PSMA is further increased in metastatic and castration-resistant carcinomas8
Indicator of poor prognosis
There is a strong positive correlation between PSMA expression and Gleason score, and PSMA can be an independent predictor of poor prognosis2,5,9
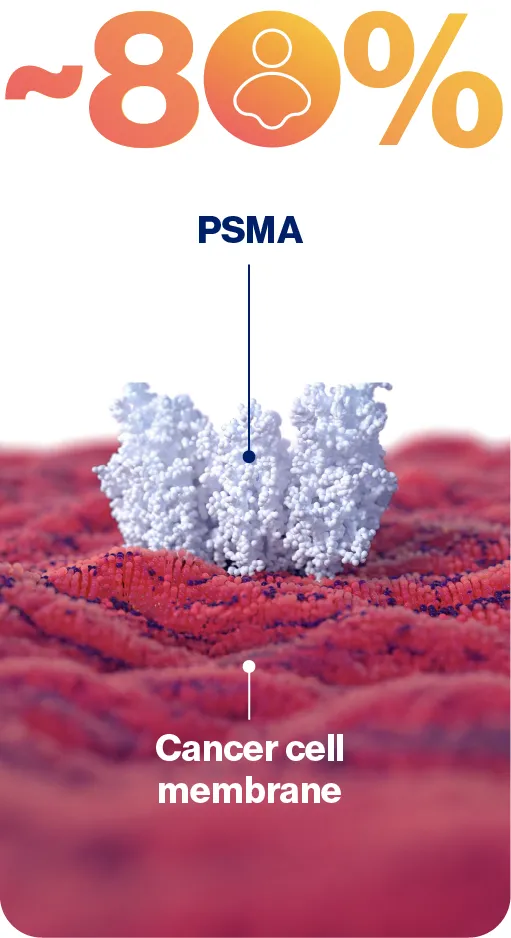
PSMA is used as a diagnostic biomarker as it is highly expressed in a large percentage of prostate cancer patients5,9
How PSMA scanning works
PSMA can be detected using non-invasive and accurate diagnostic tests such as PSMA positron emission tomography/computed tomography (PSMA PET/CT) imaging.5,10,11
How does PET/CT imaging work to detect PSMA?
Learn more about prostate cancer
Learn more about neuroendocrine tumours
*PSMA expression was assessed by immunohistochemistry and categorised according to its intensity as score 0 (no expression), 1 (low expression), 2 (medium expression), and 3 (high expression) by two independent pathologists.2
CT, computed tomography; DNA, deoxyribonucleic acid; HCP, healthcare professional; PET, positron emission tomography; PSMA, prostate-specific membrane antigen.
References
James ND, et al. Lancet 2024;403:1683–1722.
Hupe MC, et al. Front Oncol 2018;8:623.
Pomykala KL, et al. J Nucl Med 2020;61(3):405–411.
Rahbar K, et al. Mol Imag 2018;17:1536012118776068.
Cimadamore A, et al. Front Oncol 2018;8:653.
Chang SS. Rev Urol 2004;6(suppl 10):S13–8.
Fendler WP, et al. J Nucl Med 2017; 58:1196–200.
Hofman MS, et al. Lancet Oncol 2018;19:825–33.
Sartor O, et al. N Eng J Med 2021;385(12):1091–1103.
Calais J, et al. Lancet Oncol 2019;20(9):1286–1294.
Hofman MS, et al. Lancet 2020;395(10231):1208–1216 and supplementary appendix.
Schwarzenboeck S, et al. J Nucl Med 2017;58(10):1545–1552.
UK | December 2025 | FA-11482995-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.



