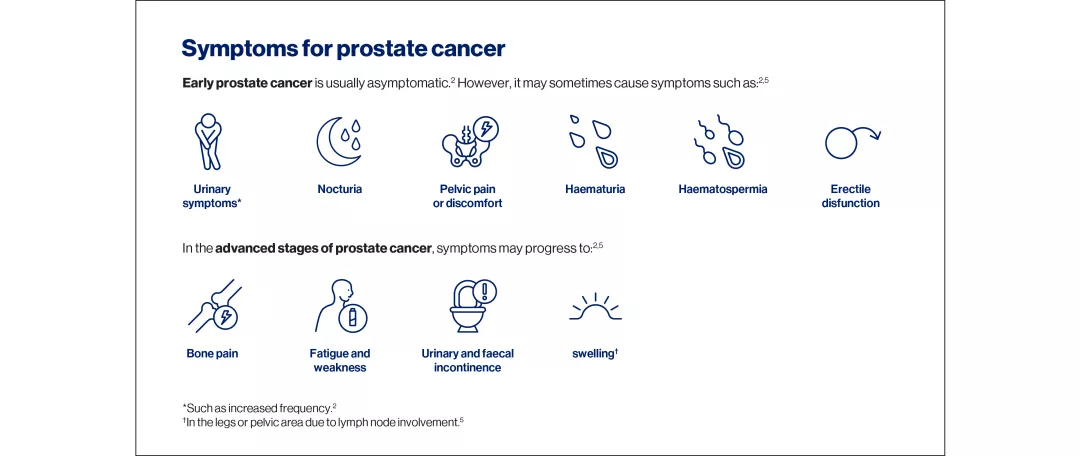
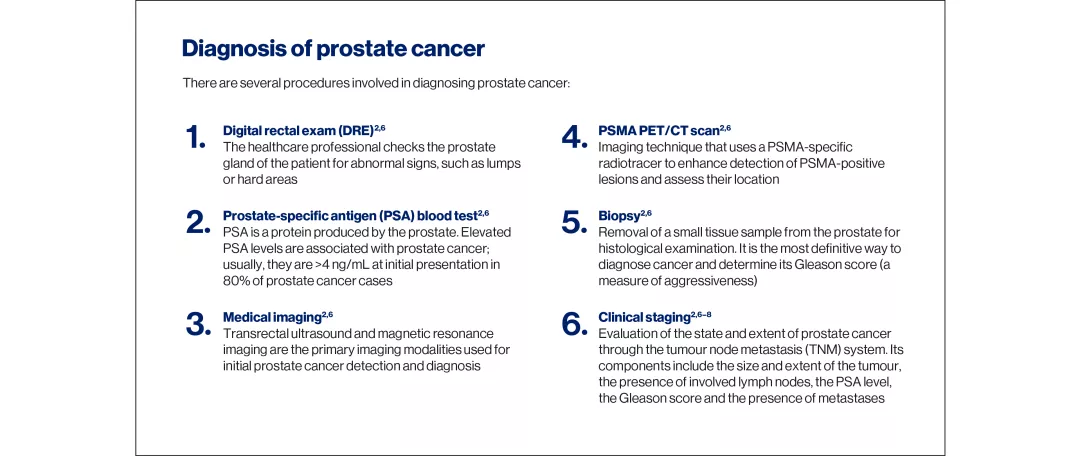
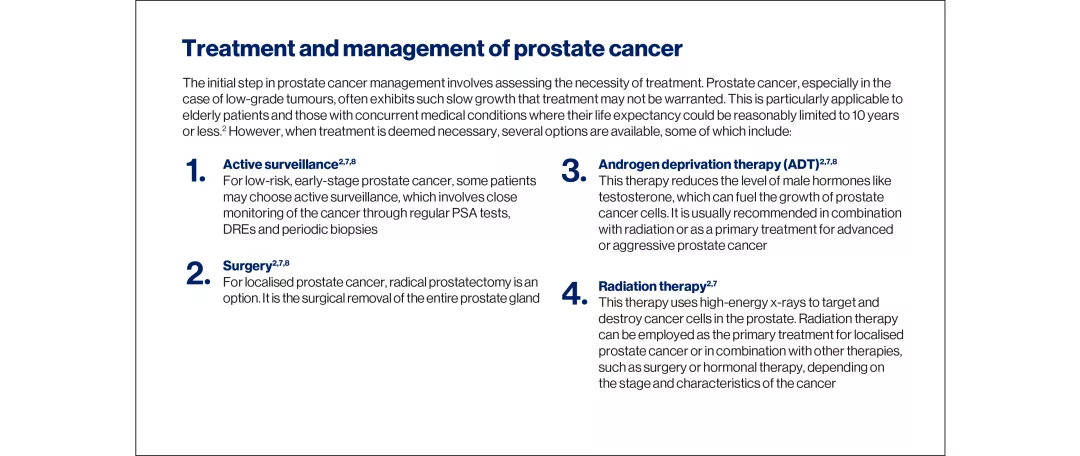
Understanding prostate cancer
Prostate cancer is a malignancy that originates in the prostate. The development of prostate cancer is linked to somatic mutations in the genetic material of prostate epithelial cells.1
The burden of prostate cancer

Prostate cancer is the most frequently diagnosed cancer in men worldwide.

In 2020, there were approximately 1,414,249 newly diagnosed cases of prostate cancer.2

Though prostate cancer progresses slowly, it is still the sixth leading cause of cancer death among men worldwide.2
This imposes a considerable medical burden due to the potential overtreatment and inadequate therapy options for advanced cases.2,3
Understanding prostate cancer
The development of prostate cancer is linked to somatic mutations in the genetic material of prostate epithelial cells. In some cases, disease progression following treatment can be attributed to intrinsic cell characteristics or acquired resistance mechanisms.1




Identifying the progression of prostate cancer
Disease progression in prostate cancer impacts the survival and quality of life of patients.2,8,9 The identification of the progression is essential to help improve patient outcomes, as it may offer an opportunity to refine treatment approaches.2 In clinical practice, there are several factors to consider when determining the timing for therapeutic adjustments:2,8–10

Extent and severity of the cancer using the TNM staging system8

Biochemical recurrence9

Cancer-related symptoms2

Clinician judgment and expertise of the healthcare provider10
Regular follow-ups with a clinician are crucial in prostate cancer care to allow the early identification and monitoring of disease progression and side effects. Through routine clinical assessments, PSA tests, imaging and physical examinations, clinicians can track the changes in cancer status and tailor treatment, if necessary.2,8,10
Timely follow-up is vital in optimising outcomes and ensuring the most appropriate management of prostate cancer, providing the best quality of care for the patient8,10
There is a growing need for more targeted and precise treatment approaches2,3
Learn more about scanning for PSMA
Learn more about neuroendocrine tumours
ADT, androgen deprivation therapy; CT, computed tomography; DRE, digital rectal exam; PET, positron emission tomography; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; TNM, tumour, node, metastasis.
References
Wasim S, et al. Int J Mol Sci 2022;23(22):14257.
Leslie SW, et al. In: StatPearls. Treasure Island (FL): StatPearls Publishing; November 13, 2023.
Wang G, et al. Genes Dev 2018;32(17–18):1105–1140.
Gann PH. Rev Urol 2022;4 Suppl 5(Supply 5):S3–S10.
Cancer Research UK. Symptoms of metastatic prostate cancer. Available at: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/metastatic-cancer/symptoms [Accessed December 2025].
Cancer Research UK. Tests for prostate cancer. Available at: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/getting-diagnosed/tests-for-prostate-cancer [Accessed December 2025].
Cancer Research UK. Treatment options for prostate cancer. Available at: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/treatment/decisions-about-your-treatment [Accessed December 2025].
Cornford P, et al. EAU–EANM–ESTRO–ESUR–SIOG guidelines on prostate cancer. Available at: https://d56bochluxqnz.cloudfront.net/documents/full-guideline/EAU-EANM-ESTRO-ESUR-ISUP-SIOG-Guidelines-on-Prostate-Cancer-2024.pdf [Accessed December 2025].
Darwish OM & Raj GV. Front Oncol 2012;2:48.
Canadian Cancer Society. Follow-up after treatment for prostate cancer. Available at: https://cancer.ca/en/cancer-information/cancer-types/prostate/treatment/follow-up [Accessed December 2025].
UK | December 2025 | FA-11482990-1
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard.