APAAACI, Asia Pacific Association of Allergy, Asthma, and Clinical Immunology; CSU, chronic spontaneous urticaria; CU-Q2oL, chronic urticaria quality of life; DLQI, dermatology life quality index; EAACI, European Academy of Allergy and Clinical Immunology; GA2LEN, Global Allergy and Asthma European Network; H1-AH, H1-antihistamine; H2-AH, H2-antihistamine; IgE, immunoglobulin E; ISS, itch severity score; LTRA, leukotriene receptor antagonist; MID, minimal important difference; OMA, omalizumab; PBO, placebo; SmPC, summary of product characteristics; UAS7, urticaria activity score (seven days).
References
Xolair® (omalizumab) Summary of Product Characteristics.
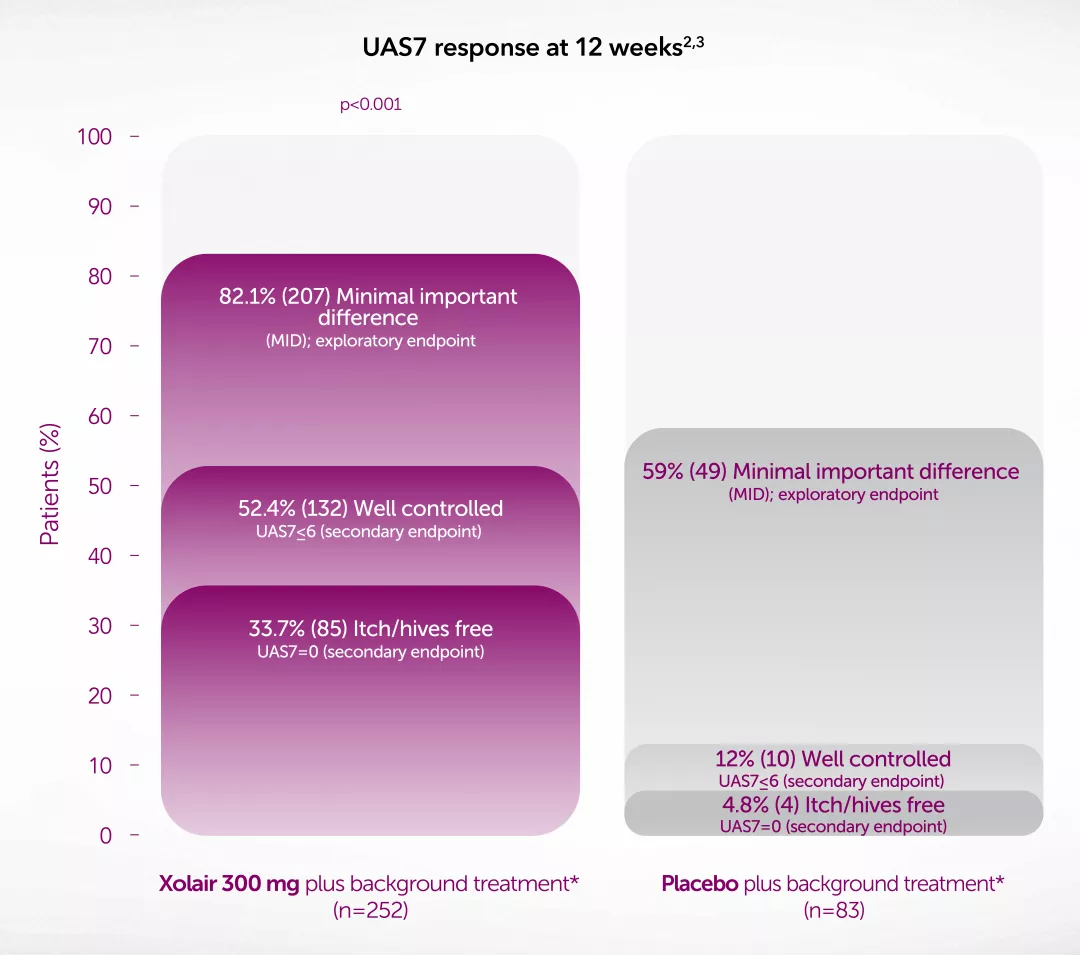
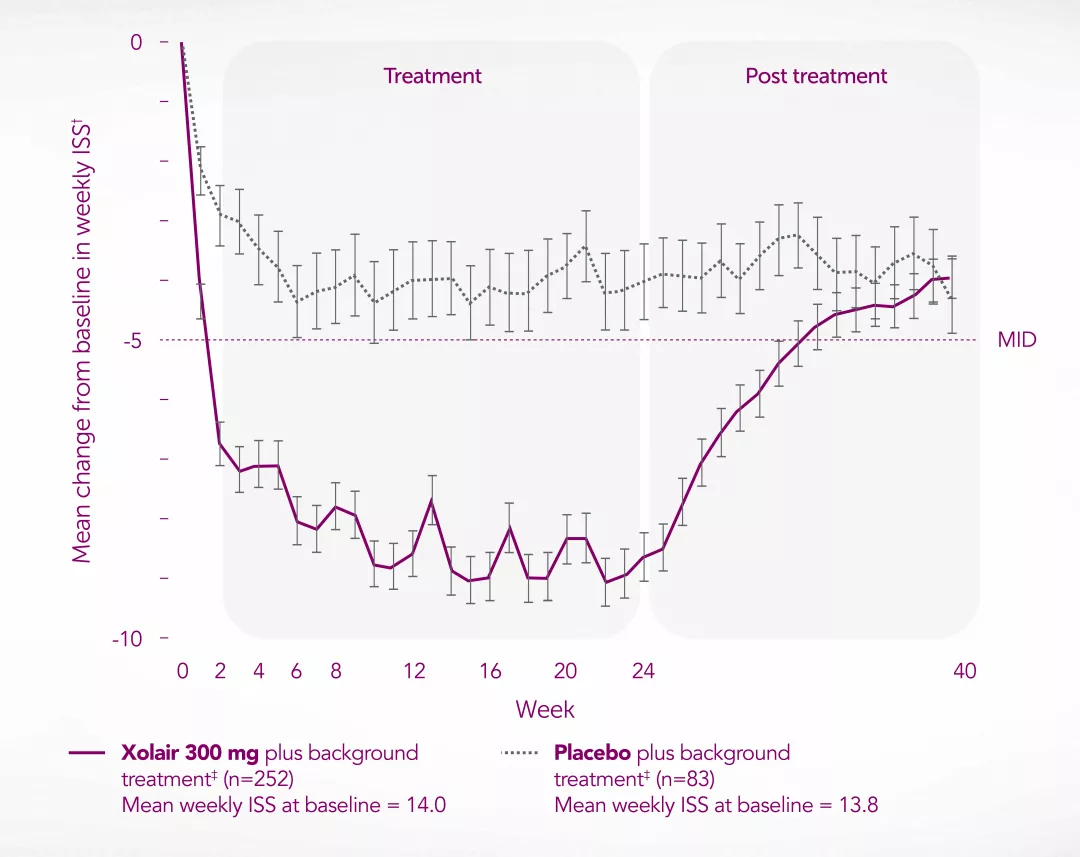
Novartis Data on File. XSU15-R002. Proportion of UAS7 MID responders at Week 12 – GLACIAL study.
Kaplan A, et al. J Allergy Clin Immunol 2013;132(1):101–109.
Zuberbier T, et al. Allergy 2022;77(3):734–766.
Finlay AY, et al. J Eur Acad Dermatol Venereol 2017;31(10):1715–1721.
Staubach P, et al. Allergy 2016;71(8):1135–1144.