

Xolair® (omalizumab) efficacy
Indications:1
Xolair is indicated as add-on therapy for the treatment of chronic spontaneous urticaria (CSU) in adult and adolescent (12 years and above) patients with inadequate response to H1 antihistamine treatment
The recommended dose of Xolair in patients with chronic spontaneous urticaria is 300 mg by subcutaneous injection every four weeks. Prescribers are advised to periodically reassess the need for continued therapy. Clinical trial experience of long-term treatment in this indication is described in section 5.1 of the SmPC.1
Please refer to the Xolair Summary of Product Characteristics (SmPC) for the full therapeutic indication.1
In patients with CSU, the most common adverse reactions that occurred during treatment with Xolair were sinusitis (4.9%), headache (6.1%), arthralgia (2.9%), injection site reaction (2.7%) and upper respiratory tract infections (5.7%). Please see the SmPC for the full safety profile information.
Click here for more information on the safety profile of Xolair.
Xolair may help reduce occurrence of symptoms vs placebo2,3
33.7% of 252 patients on omalizumab achieved UAS7=0 vs 4.8% of 83 patients on placebo at week 12 in the GLACIAL study which evaluated efficacy as a secondary objective; p<0.001.3
The 2021 international EAACI/GA²LEN/EuroGuiDerm/APAAACI guidelines4 state that the “goal of treatment is to treat the disease until it is gone [...] aiming at a continuous UAS7=0, complete control and a normalization of quality of life.”
It is imperative that patients are monitored regularly from diagnosis, to establish their level of disease control, so that treatment can be optimised accordingly. Find out more and download monitoring tools here.
See the difference that Xolair could make for your CSU patients in the ‘THINK’ sections below.
At 12 weeks (Xolair: n=252; placebo n=83):
What is UAS7?
A gold standard in monitoring CSU
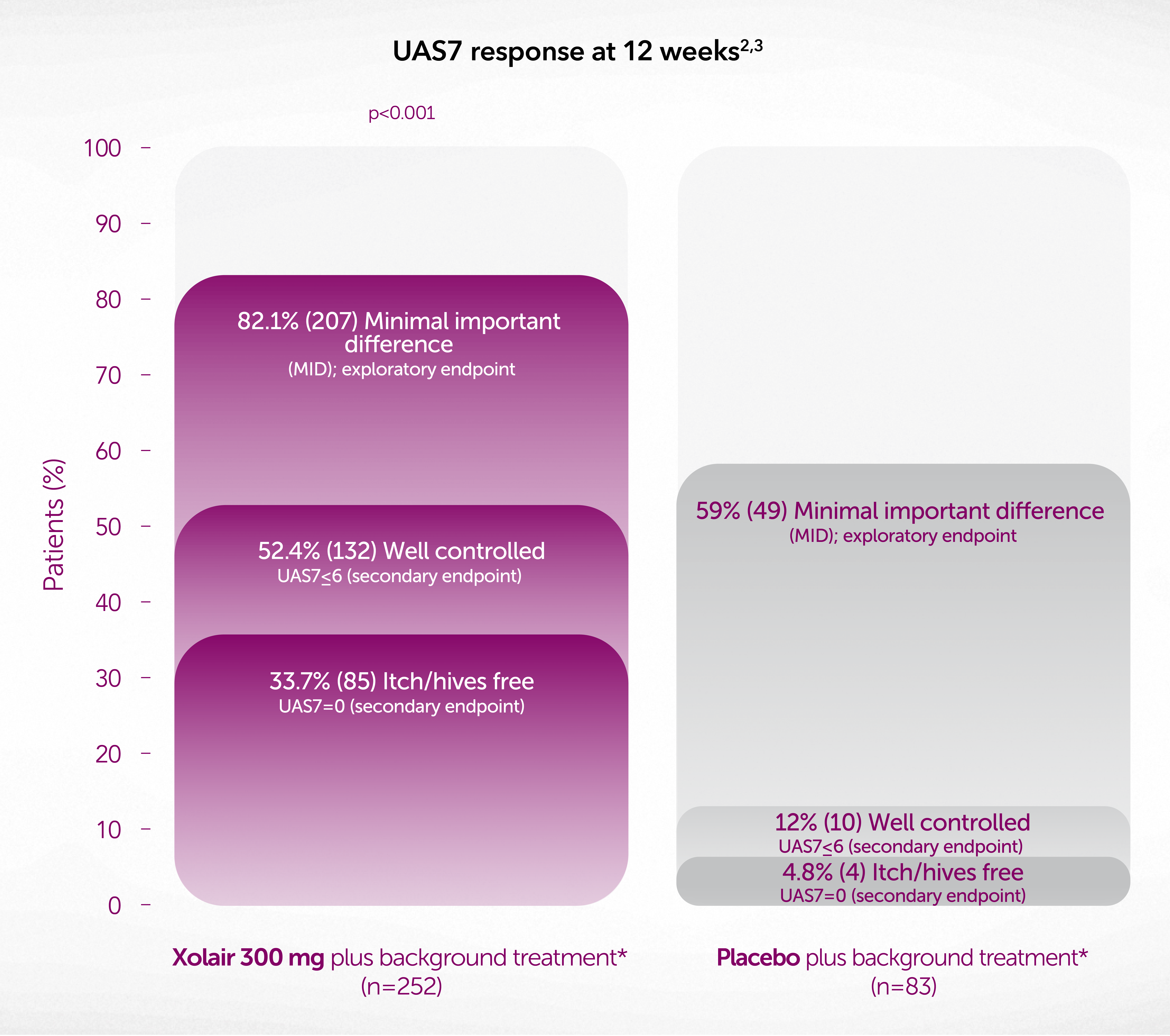
Adapted from Novartis data on File2 and Kaplan A, et al. 2013.3
Data from a randomised, double-blind, placebo-controlled, parallel group study of 336 patients aged 12–75 years with CSU for ≥6 months, itch and hives for >6 consecutive weeks before enrolment despite therapy with H1-AH ± H2-AH ± LTRAs, and a UAS7 ≥16. The primary objective was to assess the safety of Xolair compared to placebo; the overall incidence and severity of adverse events and severe adverse events were similar in both the treatment groups.
*Background treatment with H1-AH at up to 4x the approved dose (not licensed for CSU) ± H2-AH ± LTRAs. Novartis does not condone the off-label use of any medicines. Please refer to the individual products’ Summary of Product Characteristics before prescribing.
During the treatment period (24 weeks):
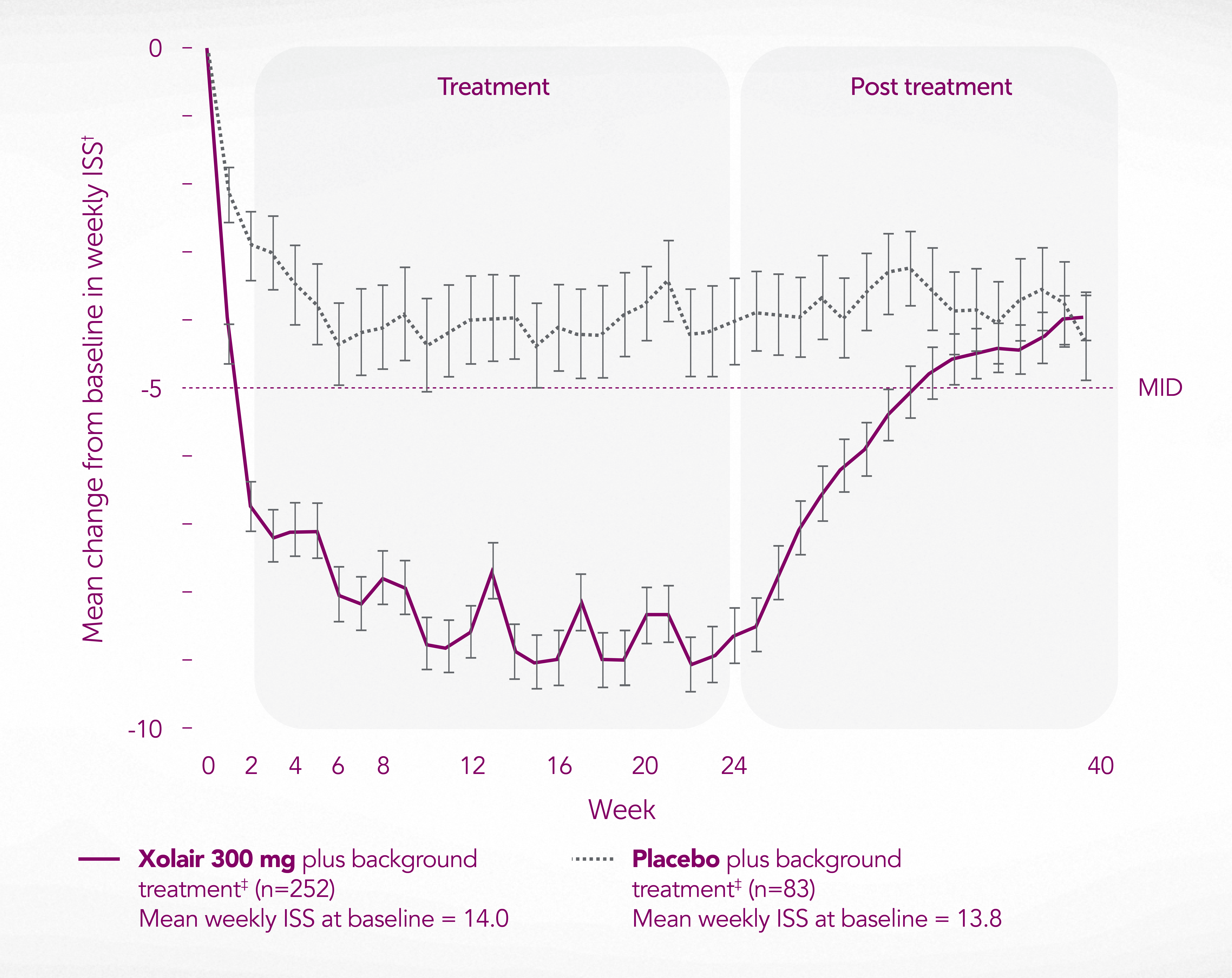
Adapted from Kaplan A, et al. 2013.3
Data from a randomised, double-blind, placebo-controlled, parallel group study of 336 patients aged 12–75 years with CSU for ≥6 months, itch and hives for >6 consecutive weeks before enrolment despite therapy with H1-AH ± H2-AH ± LTRAs, and a UAS7 ≥16.
*69.8% (N=176/252) of Xolair-treated patients achieved a reduction in ISS from baseline of ≥5 points vs 39.8% (N=33/83) of patients receiving placebo (p<0.001).
†Change from baseline to Week 12 in weekly ISS (secondary endpoint). Exploratory endpoints were also evaluated at Week 24.
‡Background treatment with H1-AH at up to 4x the approved dose (not licensed for CSU) ± H2-AH ± LTRAs. Novartis does not condon the off-label use of any medicines. Please refer to individual products’ Summary of Product Characteristics before prescribing.
Reduced occurrence of angioedema vs placebo6
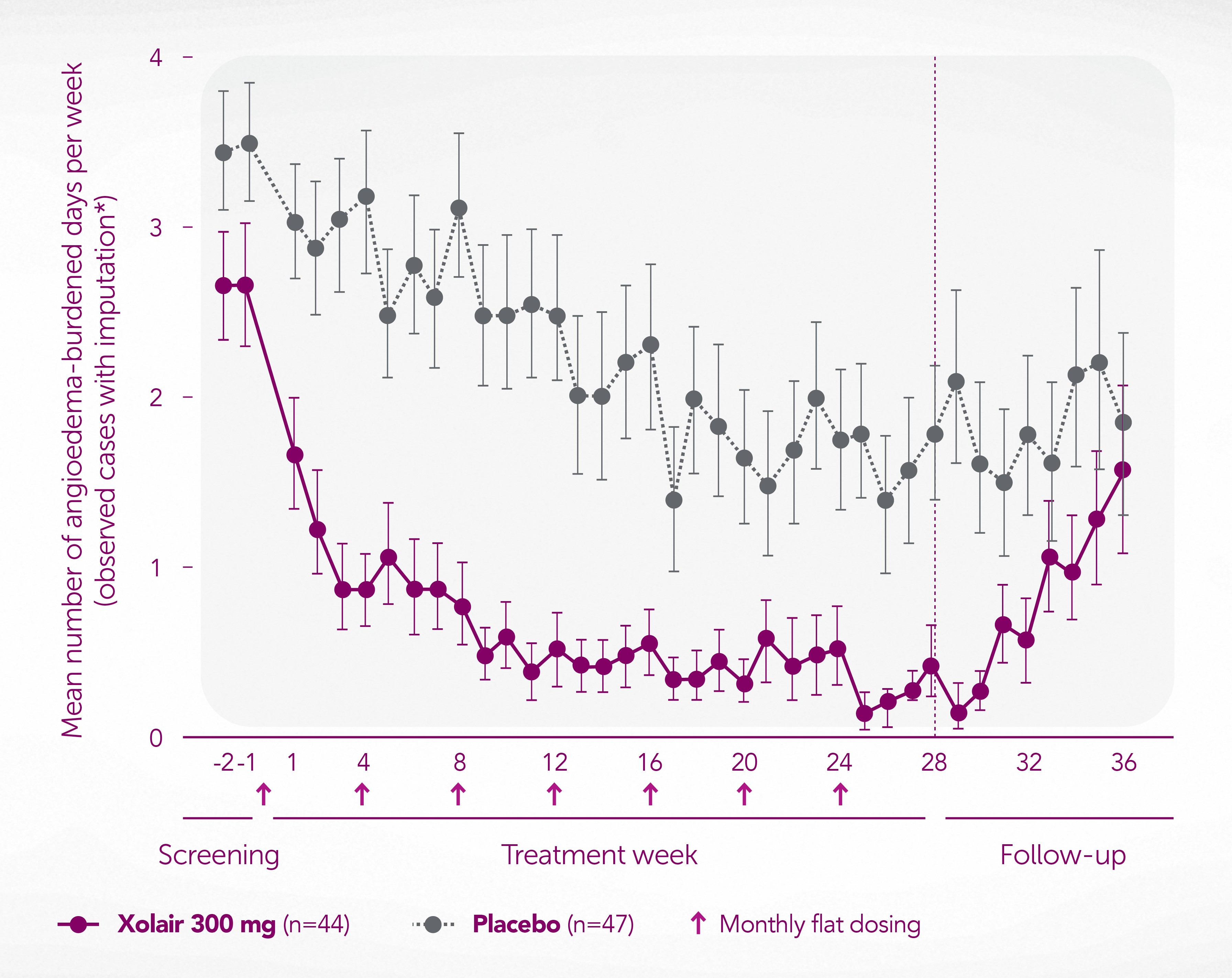
Adapted from Staubach P, et al. 2016.6
Data from a randomised, double-blind, placebo-controlled study of 91 patients with CSU aged 18–75 years with wheals and ≥4 occurrences of angioedema in the last 6 months who were symptomatic despite therapy with high-dose H1-AH (2–4x the approved dose), and a UAS7 ≥14. Xolair was significantly superior to placebo in CU-Q2oL total score from Week 4 onwards over 28 weeks (p<0.001, primary endpoint). Novartis does not condone the off-label use of any medicines. Please refer to individual products’ Summary of Product Characteristics before prescribing.
*Imputation: If for 5 or 6 days angioedema was assessed – no angioedema was assumed for the days without assessment. Full analysis set.
Global, multicentre, randomised, double-blind, placebo-controlled study investigating Xolair in CSU patients whose signs and symptoms persisted despite treatment with H1-AH at up to 4 times the approved dose* ± H2-AH ± LTRAs
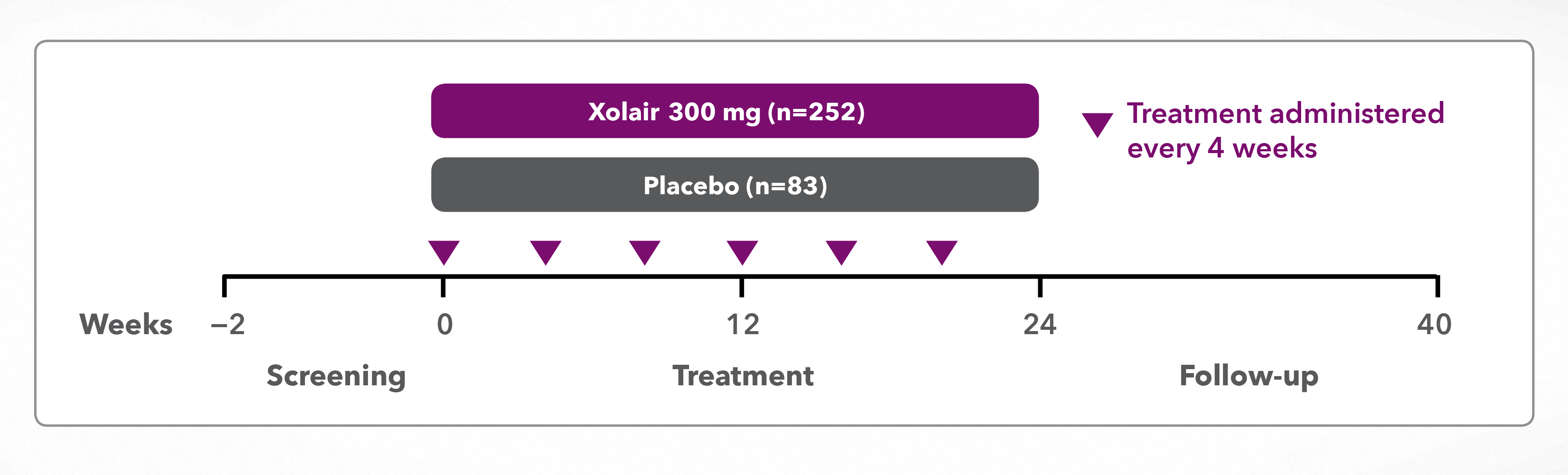
*Antihistamine dose is not licenced for CSU. Novartis does not condone the off-label use of any medicines. Please refer to individual products’ Summary of Product Characteristics before prescribing.
Randomised, double-blind, placebo-controlled, multicentre, 28-week treatment study with an 8-week follow-up period
*Antihistamine dose is not licenced for CSU. Novartis does not condone the off-label use of any medicines. Please refer to individual products’ Summary of Product Characteristics before prescribing.
Contact us now to arrange a tailored discussion, or learn more via the recommended link below.
Discover resources for you and your patients
APAAACI, Asia Pacific Association of Allergy, Asthma, and Clinical Immunology; CSU, chronic spontaneous urticaria; CU-Q2oL, chronic urticaria quality of life; DLQI, dermatology life quality index; EAACI, European Academy of Allergy and Clinical Immunology; GA2LEN, Global Allergy and Asthma European Network; H1-AH, H1-antihistamine; H2-AH, H2-antihistamine; IgE, immunoglobulin E; ISS, itch severity score; LTRA, leukotriene receptor antagonist; MID, minimal important difference; OMA, omalizumab; PBO, placebo; SmPC, summary of product characteristics; UAS7, urticaria activity score (seven days).
References
Xolair® (omalizumab) Summary of Product Characteristics.
Novartis Data on File. XSU15-R002. Proportion of UAS7 MID responders at Week 12 – GLACIAL study.
Kaplan A, et al. J Allergy Clin Immunol 2013;132(1):101–109.
Zuberbier T, et al. Allergy 2022;77(3):734–766.
Finlay AY, et al. J Eur Acad Dermatol Venereol 2017;31(10):1715–1721.
Staubach P, et al. Allergy 2016;71(8):1135–1144.
UK | May 2025 | FA-11222288
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard. Adverse events should also be reported to Novartis online through the pharmacovigilance intake (PVI) tool at www.novartis.com/report, or alternatively email [email protected] or call 01276 698370.





