

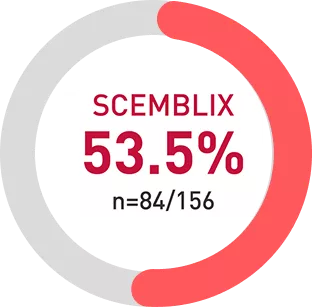
2.7x more patients stayed on treatment vs TKI at week 961
More than half of patients stayed on SCEMBLIX through the Week 96 analysis vs bosutinib1


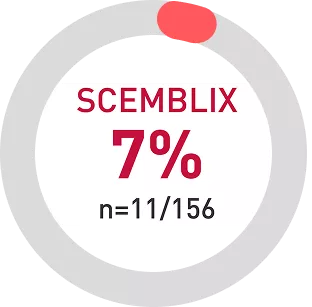
Discontinuation rate due to AEs was nearly 3.5x times lower with SCEMBLIX vs bosutinib1


42.3% of patients on SCEMBLIX had an AE that required dose reduction/interruption vs 64.5% on bosutinib1
By week 48, 47% in Scemblix arm reported Improvement in CML symptoms since starting treatment4
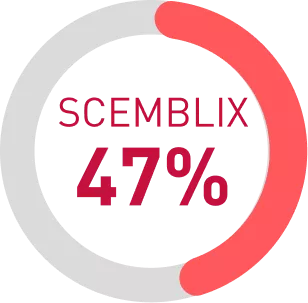

The PGIC-CML is a single question assessing patient self-report of change in CML symptom severity since the start of treatment.4
Considering all patients randomized on each arm (N = 157 asciminib and N = 76 bosutinib), Of note, very few patients on either treatment arm (n=6, <4%) reported any worsening of their CML symptoms at any time point.4
Objective: we report the long term impact on CML-specific symptoms, HRQOL, and work productivity in patients with CML-CP treated with asciminib compared to those treated with bosutinib up to 48 weeks of treatment.4
AEs, Adverse events; TKI, Tyrosine Kinase Inhibitor
For Scemblix 20mg and 40mg Abbreviated Prescription Information
For Scemblix 20mg and 40mg Abbreviated Prescription Information
References
Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, Apperley JF, Lomaia E, Voloshin S, Turkina A, Kim DW. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023 Mar;37(3):617-26.
Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, Apperley JF, Lomaia E, Voloshin S, Turkina A, Kim DW. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL SUPPLEMENT. Leukemia. 2023 Mar;37(3):617-26.
SCEMBLIX Summary of product characteristics (SMPC). Available at: https://www.medicines.org.uk/emc/product/13818/smpc/print . Last accessed 16/6/2025
Réa D, Boquimpani C, Mauro MJ, Minami Y, Allepuz A, Maheshwari VK, D’Alessio D, Wu Y, Lawrance R, Narbutas S, Sharf G. Health-related quality of life of patients with resistant/intolerant chronic phase chronic myeloid leukemia treated with asciminib or bosutinib in the phase 3 ASCEMBL trial. Leukemia. 2023 Apr 14:1-8.
Réa D, Boquimpani C, Mauro MJ, Minami Y, Allepuz A, Maheshwari VK, D’Alessio D, Wu Y, Lawrance R, Narbutas S, Sharf G. Health-related quality of life of patients with resistant/intolerant chronic phase chronic myeloid leukemia treated with asciminib or bosutinib in the phase 3 ASCEMBL trial. SUPPLEMENT. Leukemia. 2023 Apr 14:1-8.
Approved by Egyptian Drug Authority: HF0424OA4707/092025. Invalidation date: 18/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
HF0424OA4707/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
