
Hochhaus A, Réa D, Boquimpani C, Minami Y, Cortes JE, Hughes TP, Apperley JF, Lomaia E, Voloshin S, Turkina A, Kim DW. Asciminib vs bosutinib in chronic-phase chronic myeloid leukemia previously treated with at least two tyrosine kinase inhibitors: longer-term follow-up of ASCEMBL. Leukemia. 2023 Mar;37(3):617-26.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. 2021 Nov 25;138(21):2031-41.
European Medicine Association (EMA). Scemblix Assessment report. Available at: https://www.ema.europa.eu/en/documents/assessment-report/scemblix-epar-public-assessment-report_en.pdf .Last accessed 16/6/2025
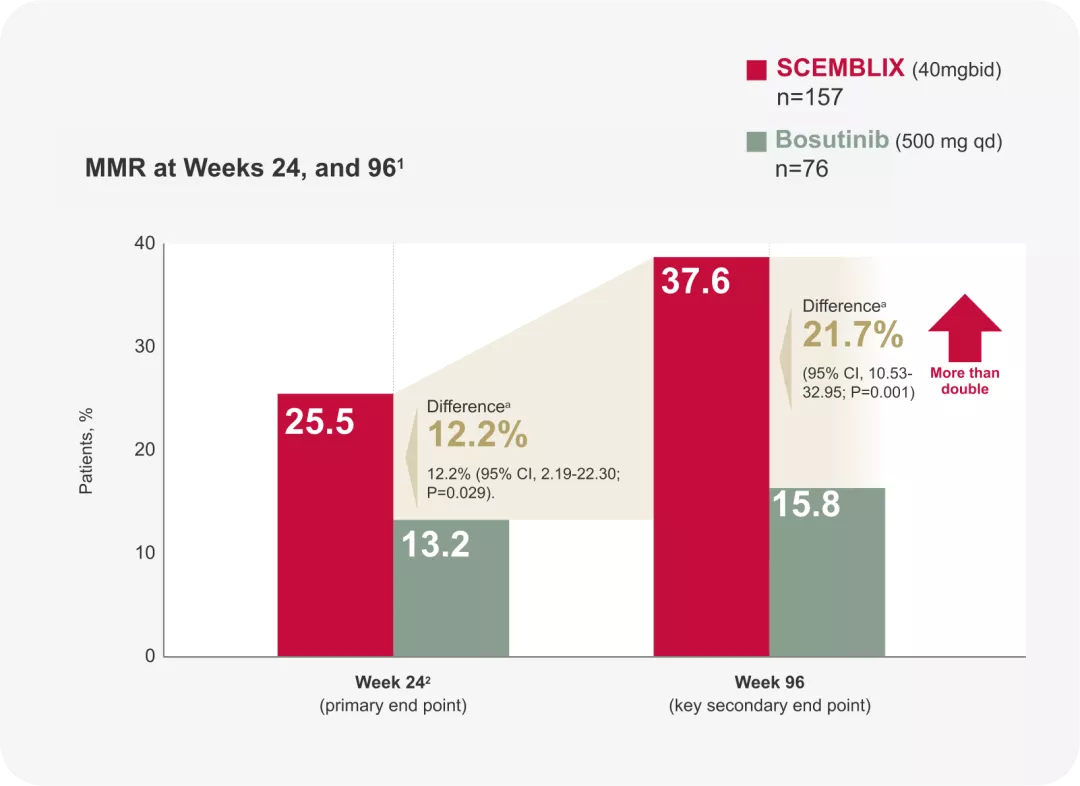
MMR at 24 weeks
MMR at 96 weeks
Cytogenetic response rates and MMR rates at and by scheduled data collection time points
Time to and duration of MMR
Time to and duration of CCyR
Safety Profile and tolerability
Time to treatment failure
Progression-free survival
Overall survival
Pharmacologic parameters
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. 2021 Nov 25;138(21):2031-41.
Rea D, Mauro MJ, Boquimpani C, Minami Y, Lomaia E, Voloshin S, Turkina A, Kim DW, Apperley JF, Abdo A, Fogliatto LM. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood, The Journal of the American Society of Hematology. SUPPLEMENT. 2021 Nov 25;138(21):2031-41.

More than Double the chance of achieving MMR than TKI's, as early as 12 weeks3 and durable to 96 weeks, higher percentage of patients achieving MR4 or Deeper1
EARLY, DURABLE, and deeper responses vs bosutinib2
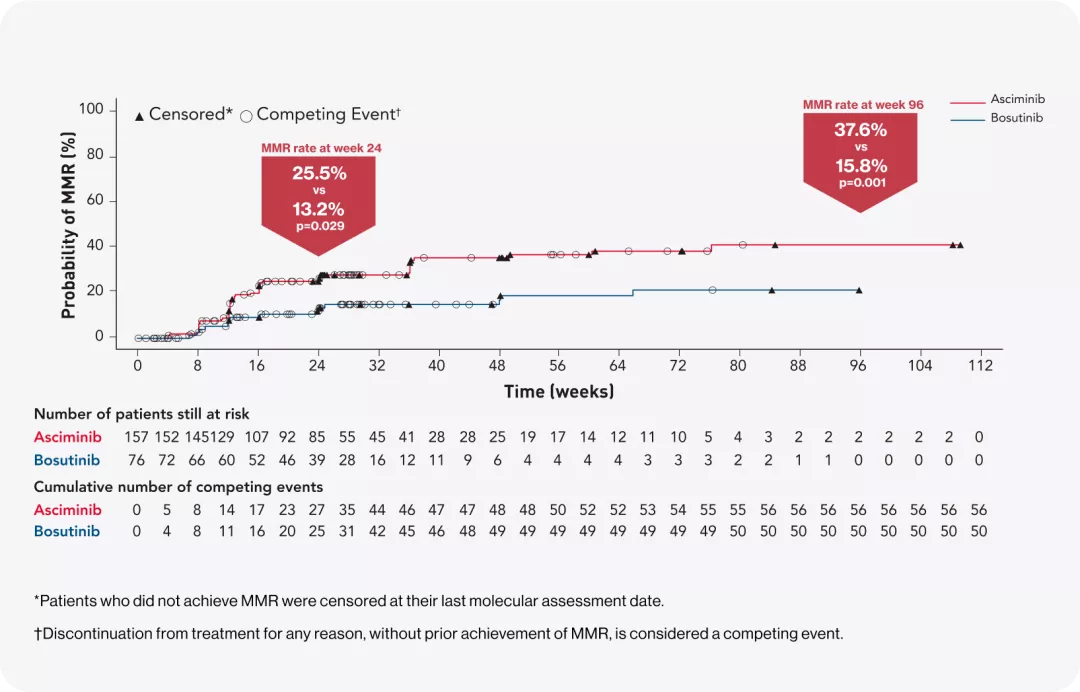
EARLY responses as early as week 123 and demonstrated durability over 96 weeks.1
MMR rates: MORE THAN DOUBLE the increase in MMR benefit1
CCyR rates: more patients who received SCEMBLIX achieved CCyR over time vs Bosutinib
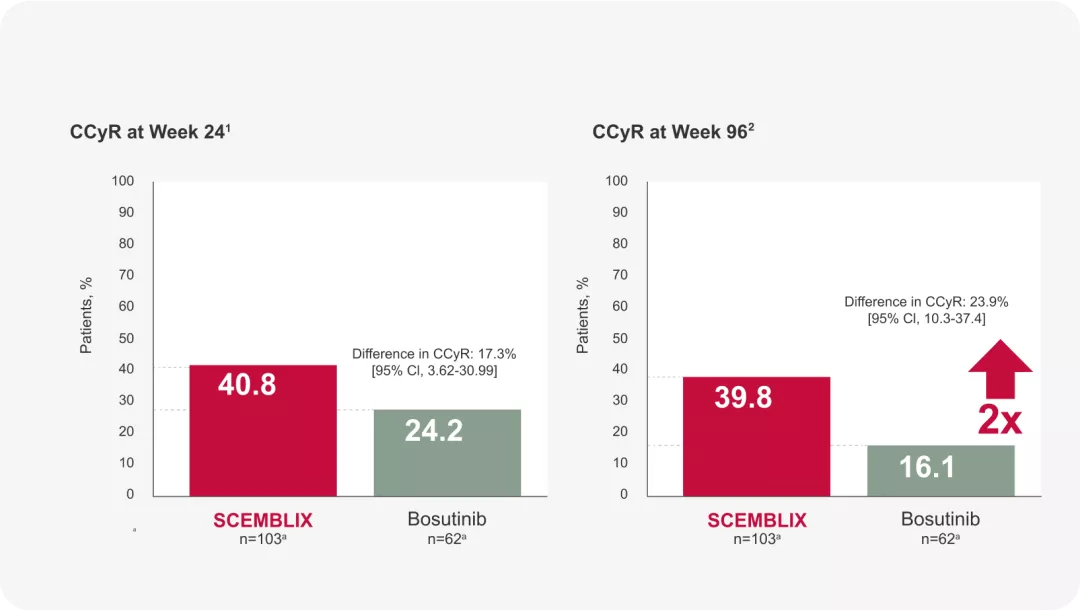
SCEMBLIX produced significantly better CCyR rates vs bosutinib, at Weeks 242 and 961
DMR rates: Deep Molecular Response rates* At week 961
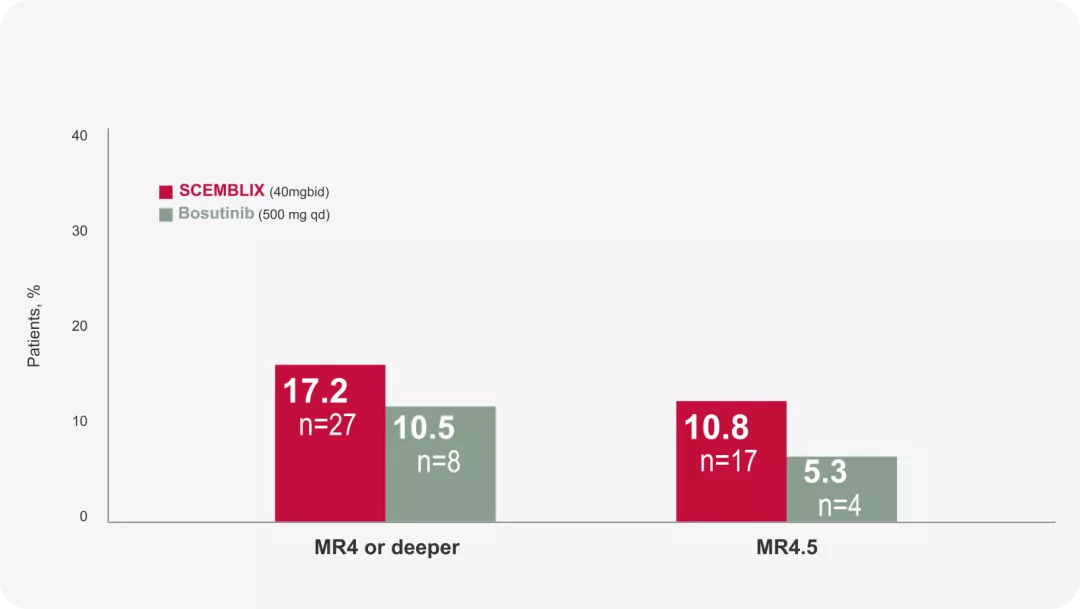
*(MR4, BCR::ABL1IS ≤ 0.01%; and MR4.5, BCR::ABL1IS ≤ 0.0032%)
References
ASCEMBL: a phase 3 trial to evaluate the efficacy and safety of a STAMP inhibitor in CML1
Study design and key eligibility criteria1
Multicentre, randomised, active-controlled and open-label Phase 3 study1

Primary endpoint1
Key secondary endpoint1
Other select secondary endpoints2
*Must meet the definition of treatment failure per the 2013 European Leukaemia Net recommendations. Patients meeting treatment failure criteria on bosutinib could be switched to asciminib (SCEMBLIX).
† Defined as nonhaematologic grade 3 or 4 toxicity while on therapy, persistent grade 2 toxicity, unresponsive to optimal management, including dose adjustments; or haematologic grade 3 or 4 toxicity while on therapy, recurrent after dose reduction to the lowest recommended dose.
‡ Patients will continue to receive study treatment for up to 96 weeks after the last patient’s first dose or 48 weeks after the last patient switches to asciminib (SCEMBLIX), whichever is longer.
CML, chronic myeloid leukemia; ABL 1, Abelson protein tyrosine kinase 1; TKI, Tyrosine Kinase Inhibitor; BCR, breakpoint cluster region; IS, International Scale; STAMP, specifically targeting the ABL1 myristoyl pocket; MMR, Major molecular response; CCyR, complete cytogenetic response; DMR, Deep molecular response.
References
For Scemblix 20mg and 40mg Abbreviated Prescription Information
For Scemblix 20mg and 40mg Abbreviated Prescription Information
Approved by Egyptian Drug Authority: HF0424OA4707/092025. Invalidation date: 18/09/2027.
Kindly report any violated online promotional, educational and awareness material not having this message to The General administration for Regulation of Marketing & Advertising Materials at:
www.edaegypt.gov.eg
Image

|
HF0424OA4707/092025 |
Adverse Events Reporting We encourage using the following Electronic reporting tool for reporting into the safety database directly: |
